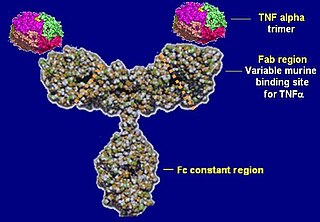
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.

Hypersensitivity is an abnormal physiological condition in which there is an undesirable and adverse immune response to antigen. It is an abnormality in the immune system that causes immune diseases including allergies and autoimmunity. It is caused by many types of particles and substances from the external environment or from within the body that are recognized by the immune cells as antigens. The immune reactions are usually referred to as an over-reaction of the immune system and they are often damaging and uncomfortable.
Daclizumab is a therapeutic humanized monoclonal antibody which was used for the treatment of adults with relapsing forms of multiple sclerosis (MS). Daclizumab works by binding to CD25, the alpha subunit of the IL-2 receptor of T-cells.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
Omalizumab, sold under the brand name Xolair among others, is an injectable medication to treat severe persistent allergic forms of asthma, nasal polyps, urticaria (hives), and immunoglobulin E-mediated food allergy.
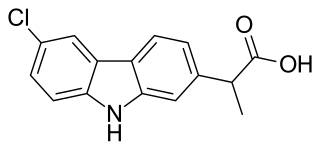
Carprofen is a nonsteroidal anti-inflammatory drug (NSAID) of the carbazole and propionic acid class that was previously for use in humans and animals but is now only available to veterinarians for prescribing as a supportive treatment for various conditions in animals. Carprofen reduces inflammation by inhibition of COX-1 and COX-2; its specificity for COX-2 varies from species to species. Marketed under many brand names worldwide, carprofen is used as a treatment for inflammation and pain, including joint pain and postoperative pain.
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.

Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for non-Hodgkin's lymphoma. The drug uses the monoclonal mouse IgG1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.
Discoid lupus erythematosus (DLE) is an uncommon autoimmune disease of the basal cell layer of the skin. It occurs in humans and cats, more frequently occurring in dogs. It was first described in dogs by Griffin and colleagues in 1979. DLE is one form of cutaneous lupus erythematosus (CLE). DLE occurs in dogs in two forms: a classical facial predominant form or generalized with other areas of the body affected. Other non-discoid variants of CLE include vesicular CLE, exfoliative CLE and mucocutaneous CLE. It does not progress to systemic lupus erythematosus (SLE) in dogs. SLE can also have skin symptoms, but it appears that the two are either separate diseases. DLE in dogs differs from SLE in humans in that plasma cells predominate histologically instead of T lymphocytes. Because worsening of symptoms occurs with increased ultraviolet light exposure, sun exposure most likely plays a role in DLE, although certain breeds (see below) are predisposed. After pemphigus foliaceus, DLE is the second most common autoimmune skin disease in dogs.

Interleukin-31 (IL-31) is a protein that in humans is encoded by the IL31 gene that resides on chromosome 12. IL-31 is an inflammatory cytokine that helps trigger cell-mediated immunity against pathogens. It has also been identified as a major player in a number of chronic inflammatory diseases, including atopic dermatitis.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
Lebrikizumab, sold under the brand name Ebglyss is a humanized monoclonal antibody used for the treatment of atopic dermatitis.

Secukinumab, sold under the brand name Cosentyx among others, is a human IgG1κ monoclonal antibody used for the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis. It binds to the protein interleukin (IL)-17A and is marketed by Novartis.

Tralokinumab sold under the brand names Adtralza (EU/UK) and Adbry (US) among others, is a human monoclonal antibody used for the treatment of atopic dermatitis. Tralokinumab targets the cytokine interleukin 13.

Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as atopic dermatitis (eczema), asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.
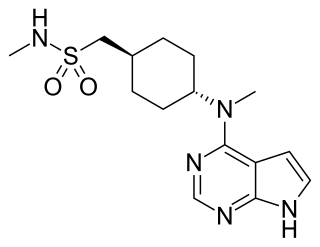
Oclacitinib, sold under the brand name Apoquel among others, is a veterinary medication used in the control of atopic dermatitis and pruritus from allergic dermatitis in dogs at least 12 months of age. Chemically, it is a synthetic cyclohexylamino pyrrolopyrimidine janus kinase inhibitor that is relatively selective for JAK1. It inhibits signal transduction when the JAK is activated and thus helps downregulate expression of inflammatory cytokines.
Non-surgical fertility control is the prevention of reproduction without the use of surgery. The most common form of sterilization in dogs and cats is surgical, spaying in females and castration in males. Non-surgical fertility control can either result in sterilization or temporary contraception and could offer a cheaper way to keep wild dog and cat populations under control. As of 2019, only contraceptives are commercially available. Research is ongoing into methods that could result in permanent suppression of fertility.
Frunevetmab, sold under the brand name Solensia, is a monoclonal antibody used to treat pain associated with osteoarthritis in cats. It is the first monoclonal antibody drug approved by the US Food and Drug Administration for animal use. Frunevetmab is the international nonproprietary name.
Bedinvetmab, sold under the brand name Librela is a canine monoclonal antibody used for the control of pain associated with osteoarthritis in dogs. Librela is sponsored by Zoetis.










