
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral:
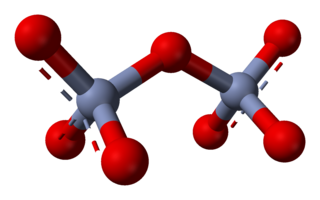
Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
Zinc carbonate is the inorganic compound with the formula ZnCO3. It is a white solid that is insoluble in water. It exists in nature as the mineral smithsonite. It is prepared by treating cold solutions of zinc sulfate with potassium bicarbonate. Upon warming, it converts to basic zinc carbonate (Zn5(CO3)2(OH)6).
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Carl Heinrich Hermann, or Carl HermannGerman:[kaʁlˈhɛʁman], was a German physicist and crystallographer known for his research in crystallographic symmetry, nomenclature, and mathematical crystallography in N-dimensional spaces. Hermann was a pioneer in crystallographic databases and, along with Paul Peter Ewald, published the first volume of the influential Strukturbericht in 1931.
Lead(II) carbonate is the chemical compound with the chemical formula PbCO3. It is a white solid with several practical uses, despite its toxicity. It occurs naturally as the mineral cerussite.
Silver sulfate is the inorganic compound with the formula Ag2SO4. It is a white solid with low solubility in water.
Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).
Lead(II) chromate is an inorganic compound with the chemical formula PbCrO4. It has a vivid yellow color and is generally insoluble. Two polymorphs of lead chromate are known, orthorhombic and the more stable monoclinic form. Monoclinic lead chromate is used in paints under the name chrome yellow, and many other names. It occurs also as the mineral crocoite.

Nickel(II) chromate (NiCrO4) is an acid-soluble compound, red-brown in color, with high tolerances for heat. It and the ions that compose it have been linked to tumor formation and gene mutation, particularly to wildlife.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.
The nitrate selenites are mixed anion compounds containing distinct nitrate (NO3−)and selenite (SO32−) groups. The compounds are colourless unless coloured by cations.

Iron(II) perchlorate is the inorganic compound with the formula Fe(ClO4)2·6H2O. A green, water-soluble solid, it is produced by the reaction of iron metal with dilute perchloric acid followed by evaporation of the solution:
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
A tellurite tellurate is chemical compound or salt that contains tellurite and tellurate anions [TeO3]2- [TeO4 ]2-. These are mixed anion compounds, meaning the compounds are cations that contain one or more anions. Some have third anions. Environmentally, tellurite [TeO3]2- is the more abundant anion due to tellurate's [TeO4 ]2- low solubility limiting its concentration in biospheric waters. Another way to refer to the anions is tellurium's oxyanions, which happen to be relatively stable.

An arsenate arsenite is a chemical compound or salt that contains arsenate and arsenite anions (AsO33- and AsO43-). These are mixed anion compounds or mixed valence compounds. Some have third anions. Most known substances are minerals, but a few artificial arsenate arsenite compounds have been made. Many of the minerals are in the Hematolite Group.
Nickel(II) selenate is a selenate of nickel with the chemical formula NiSeO4.
Caesium selanate is an inorganic compound, with the chemical formula of Cs2SeO4. It can form colourless crystals of the orthorhombic crystal system.
Barium selenate is an inorganic compound with the chemical formula BaSeO4. It is isomorphous with barium sulfate, but its solubility is 18 times that of barium sulfate, and its thermal stability is worse than that of barium sulfate.














