A brain tumor occurs when a group of cells within the brain turn cancerous and grow out of control, creating a mass. There are two main types of tumors: malignant (cancerous) tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.
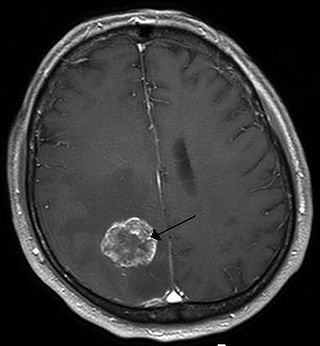
Oligodendrogliomas are a type of glioma that are believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. They occur primarily in adults but are also found in children.

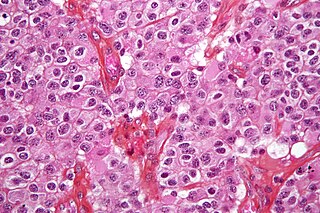
Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.

Isocitrate dehydrogenase (IDH) (EC 1.1.1.42) and (EC 1.1.1.41) is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome.
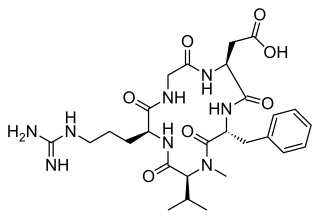
Cilengitide is a molecule designed and synthesized at the Technical University Munich in collaboration with Merck KGaA in Darmstadt. It is based on the cyclic peptide cyclo(-RGDfV-), which is selective for αv integrins, which are important in angiogenesis, and other aspects of tumor biology. Hence, it is under investigation for the treatment of glioblastoma, where it may act by inhibiting angiogenesis, and influencing tumor invasion and proliferation.

Methylated-DNA--protein-cysteine methyltransferase(MGMT), also known as O6-alkylguanine DNA alkyltransferaseAGT, is a protein that in humans is encoded by the MGMT gene. MGMT is crucial for genome stability. It repairs the naturally occurring mutagenic DNA lesion O6-methylguanine back to guanine and prevents mismatch and errors during DNA replication and transcription. Accordingly, loss of MGMT increases the carcinogenic risk in mice after exposure to alkylating agents. The two bacterial isozymes are Ada and Ogt.

A nervous system tumor is a tumor that arises within the nervous system, either the central nervous system (CNS) or the peripheral nervous system (PNS). Nervous system primary tumors include various types of brain tumor and spinal tumors, such as gliomas, and meningiomas, and schwannomas and can be either benign or malignant.

Isocitrate dehydrogenase [NADP], mitochondrial is an enzyme that in humans is encoded by the IDH2 gene.

Gliosarcoma is a rare type of glioma, a cancer of the brain that comes from glial, or supportive, brain cells, as opposed to the neural brain cells. Gliosarcoma is a malignant cancer, and is defined as a glioblastoma consisting of gliomatous and sarcomatous components. Primary gliosarcoma (PGS) is classified as a grade IV tumor and a subtype of glioblastoma multiforme in the 2007 World Health Organization classification system (GBM). Because of a lack of specific and clear diagnostic criteria, the word "gliosarcoma" was frequently used to refer to glial tumours with mesenchymal properties, such as the ability to make collagen and reticulin.
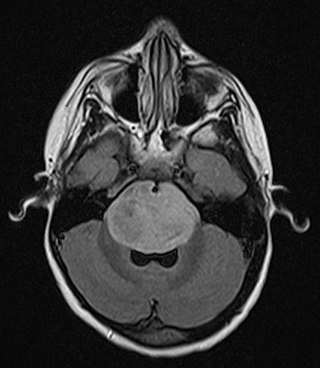
A brainstem glioma is a cancerous glioma tumor in the brainstem. Around 75% are diagnosed in children and young adults under the age of twenty, but have been known to affect older adults as well. Brainstem gliomas start in the brain or spinal cord tissue and typically spread throughout the nervous system.
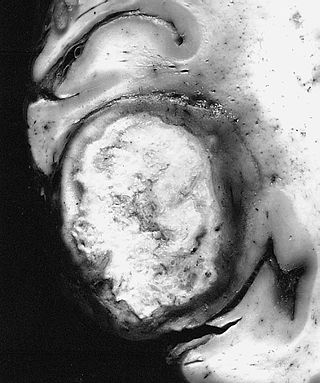
The giant-cell glioblastoma is a histological variant of glioblastoma, presenting a prevalence of bizarre, multinucleated giant cells.

Anaplastic astrocytoma is a rare WHO grade III type of astrocytoma, which is a type of cancer of the brain. In the United States, the annual incidence rate for anaplastic astrocytoma is 0.44 per 100,000 people.
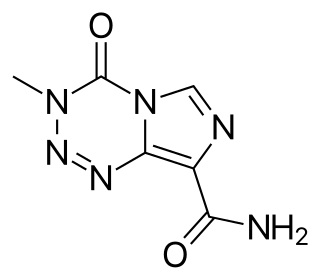
Temozolomide, sold under the brand name Temodar among others, is an anticancer medication used to treat brain tumors such as glioblastoma and anaplastic astrocytoma. It is taken by mouth or via intravenous infusion.
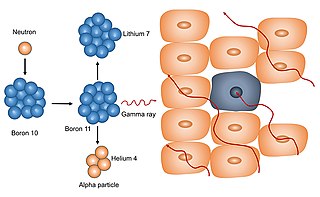
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.

Isocitrate dehydrogenase 1 (NADP+), soluble is an enzyme that in humans is encoded by the IDH1 gene on chromosome 2. Isocitrate dehydrogenases catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate. These enzymes belong to two distinct subclasses, one of which uses NAD+ as the electron acceptor and the other NADP+. Five isocitrate dehydrogenases have been reported: three NAD+-dependent isocitrate dehydrogenases, which localize to the mitochondrial matrix, and two NADP+-dependent isocitrate dehydrogenases, one of which is mitochondrial and the other predominantly cytosolic. Each NADP+-dependent isozyme is a homodimer. The protein encoded by this gene is the NADP+-dependent isocitrate dehydrogenase found in the cytoplasm and peroxisomes. It contains the PTS-1 peroxisomal targeting signal sequence. The presence of this enzyme in peroxisomes suggests roles in the regeneration of NADPH for intraperoxisomal reductions, such as the conversion of 2,4-dienoyl-CoAs to 3-enoyl-CoAs, as well as in peroxisomal reactions that consume 2-oxoglutarate, namely the alpha-hydroxylation of phytanic acid. The cytoplasmic enzyme serves a significant role in cytoplasmic NADPH production. Alternatively spliced transcript variants encoding the same protein have been found for this gene. [provided by RefSeq, Sep 2013]
Alternating electric field therapy, sometimes called tumor treating fields (TTFields), is a type of electromagnetic field therapy using low-intensity, intermediate frequency electrical fields to treat cancer. TTFields disrupt cell division by disrupting dipole alignment and inducing dielectrophoresis of critical molecules and organelles during mitosis. These anti-mitotic effects lead to cell death, slowing cancer growth. A TTField-treatment device manufactured by the Israeli company Novocure is approved in the United States and Europe for the treatment of newly diagnosed and recurrent glioblastoma, malignant pleural mesothelioma (MPM), and is undergoing clinical trials for several other tumor types. Despite earning regulatory approval, the efficacy of this technology remains controversial among medical experts.

Diffuse midline glioma, H3 K27-altered (DMG) is a fatal tumour that arises in midline structures of the brain, most commonly the brainstem, thalamus and spinal cord. When located in the pons it is also known as diffuse intrinsic pontine glioma (DIPG).

Matthias Preusser is an Austrian oncologist and Professor of Medical Oncology as well as Head of the Clinical Division of Oncology at the Medical University of Vienna. He is known for his work on neurooncology, Molecular Therapy targets and biomarkers and immunotherapy of cancer.
Anaplastic oligodendroglioma is a neuroepithelial tumor which is believed to originate from oligodendrocytes, a cell type of the glia. In the World Health Organization (WHO) classification of brain tumors, anaplastic oligodendrogliomas are classified as grade III. In the course of the disease, it can degenerate into highly malignant oligodendroglioma, grade IV. The vast majority of oligodendrogliomas occur sporadically, without a confirmed cause and without inheritance within a family.
Diffuse hemispheric glioma, H3G34 mutant (DHG) is a rare, high-grade, infiltrative WHO grade 4 brain tumor most often found in adolescents and young adults. The majority are found in the frontal, parietal, and temporal lobes.





















