In chemistry, an alkali is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases.
Soda or SODA may refer to:
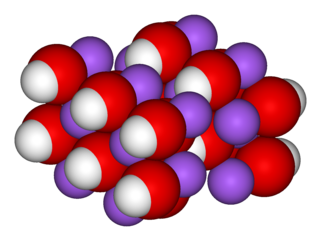
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate Na
2SiO
3, sodium orthosilicate Na
4SiO
4, and sodium pyrosilicate Na
6Si
2O
7. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
The Leblanc process was an early industrial process for making soda ash used throughout the 19th century, named after its inventor, Nicolas Leblanc. It involved two stages: making sodium sulfate from sodium chloride, followed by reacting the sodium sulfate with coal and calcium carbonate to make sodium carbonate. The process gradually became obsolete after the development of the Solvay process.
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 was estimated at 42 million tonnes, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.

Salicornia is a genus of succulent, halophytic flowering plants in the family Amaranthaceae that grow in salt marshes, on beaches, and among mangroves. Salicornia species are native to North America, Europe, Central Asia, and southern Africa. Common names for the genus include glasswort, pickleweed, picklegrass, and marsh samphire; these common names are also used for some species not in Salicornia. To French speakers in Atlantic Canada, they are known colloquially as titines de souris. The main European species is often eaten, called marsh samphire in Britain, and the main North American species is occasionally sold in grocery stores or appears on restaurant menus as sea beans, samphire greens or sea asparagus.

Samphire is a name given to a number of succulent salt-tolerant plants (halophytes) that tend to be associated with water bodies.
Saltwort is a common name for various genera of flowering plants that thrive in salty environments, typically in coastal salt marshes and seashores, including:

James Muspratt was a British chemical manufacturer who was the first to make alkali by the Leblanc process on a large scale in the United Kingdom.

Cristallo is a glass that is totally clear, without the slight yellow or greenish color originating from iron oxide impurities. This effect is achieved through small additions of manganese oxide. Often Cristallo has a low lime content, which makes it prone to glass corrosion.
Alkali manufacture is the process by which an alkali is made. Typical alkalis, produced commercially, include sodium hydroxide, sodium carbonate, potassium hydroxide and potassium carbonate.

Salsola soda, the opposite-leaved saltwort, oppositeleaf Russian thistle, or barilla plant, is a small, annual, succulent shrub that is native to the Mediterranean Basin. It is a halophyte that typically grows in coastal regions and can be irrigated with salt water.

Kali turgidum, commonly known as prickly saltwort or prickly glasswort, is an annual plant that grows in salty sandy coastal soils.

Barilla refers to several species of salt-tolerant (halophyte) plants that, until the 19th century, were the primary source of soda ash and hence of sodium carbonate. The word "barilla" was also used directly to refer to the soda ash obtained from plant sources. The word is an anglicization of the Spanish word barrilla for saltwort plants.

Alkali, or Alkaline, soils are clay soils with high pH, a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swell and difficult to clarify/settle. They derive their name from the alkali metal group of elements, to which sodium belongs, and which can induce basicity. Sometimes these soils are also referred to as alkaline sodic soils.
Alkaline soils are basic, but not all basic soils are alkaline.

Forest glass is late medieval glass produced in northwestern and central Europe from approximately 1000–1700 AD using wood ash and sand as the main raw materials and made in factories known as glasshouses in forest areas. It is characterized by a variety of greenish-yellow colors, the earlier products often being of crude design and poor quality, and was used mainly for everyday vessels and increasingly for ecclesiastical stained glass windows. Its composition and manufacture contrast sharply with Roman and pre-Roman glassmaking centered on the Mediterranean and contemporaneous Byzantine and Islamic glass making to the east.

Salicornia europaea, known as marsh samphire, common glasswort or just glasswort, is a halophytic annual dicot flowering plant in the family Amaranthaceae. Glasswort is a succulent herb also known as ‘Pickle weed’ or ‘Marsh samphire’. As a succulent, it has high water content, which accounts for its slightly translucent look and gives it the descriptive name “glasswort.” To some people, it is known as “chicken toe” because of its shape. To others, it is called “saltwort.” It grows in various zones of intertidal salt marshes, on beaches, and among mangroves.

Seidlitzia rosmarinus is a perennial-green desert species of saltwort in the Amaranthaceae family. It is endemic to the lower Jordan Valley along the Dead Sea, in Israel and Jordan, and in the Syrian desert, Central Iraq and in the coastal regions of Saudi Arabia, the islands of Bahrain, Qatar, and Iran, commonly known in Arabic by the names ʾušnān and šenān and in the Neo-Aramaic languages by reflexes of ʾuḥlā. It is often used by Bedouins for cleaning as a soap substitute. In medieval Arabic literature, it is also known by the names of "green ushnan" and "launderers' potash", having been used since time immemorial to produce nabulsi soap and as an electuary in compounding theriac for use in treating scorpion stings, as well as for extracting potassium for other medicinal uses.














