
Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, including embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.
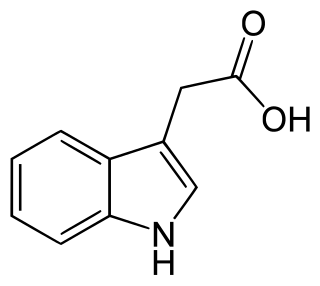
Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.

Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in cell growth and differentiation, but also affect apical dominance, axillary bud growth, and leaf senescence.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.
Shade avoidance is a set of responses that plants display when they are subjected to the shade of another plant. It often includes elongation, altered flowering time, increased apical dominance and altered partitioning of resources. This set of responses is collectively called the shade-avoidance syndrome (SAS).

Brassinolide is a plant hormone. The first isolated brassinosteroid, it was discovered when it was shown that pollen from rapeseed could promote stem elongation and cell division. The biologically active component was isolated and named brassinolide.
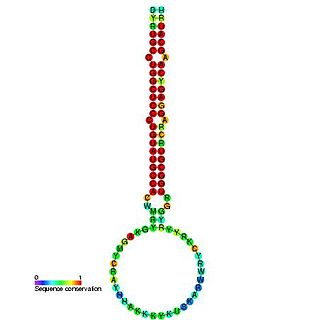
In molecular biology, mir-160 is a microRNA that has been predicted or experimentally confirmed in a range of plant species including Arabidopsis thaliana and Oryza sativa (rice). miR-160 is predicted to bind complementary sites in the untranslated regions of auxin response factor genes to regulate their expression. The hairpin precursors are predicted based on base pairing and cross-species conservation; their extents are not known. In this case, the mature sequence is excised from the 5' arm of the hairpin.
Expansins are a family of closely related nonenzymatic proteins found in the plant cell wall, with important roles in plant cell growth, fruit softening, abscission, emergence of root hairs, pollen tube invasion of the stigma and style, meristem function, and other developmental processes where cell wall loosening occurs. Expansins were originally discovered as mediators of acid growth, which refers to the widespread characteristic of growing plant cell walls to expand faster at low (acidic) pH than at neutral pH. Expansins are thus linked to auxin action. They are also linked to cell enlargement and cell wall changes induced by other plant hormones such as gibberellin, cytokinin, ethylene and brassinosteroids.
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:
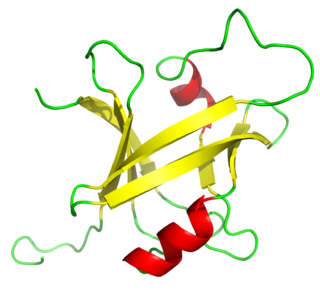
The B3 DNA binding domain (DBD) is a highly conserved domain found exclusively in transcription factors combined with other domains. It consists of 100-120 residues, includes seven beta strands and two alpha helices that form a DNA-binding pseudobarrel protein fold ; it interacts with the major groove of DNA.

Paclobutrazol (PBZ) is the ISO common name for an organic compound that is used as a plant growth retardant and triazole fungicide. It is a known antagonist of the plant hormone gibberellin, acting by inhibiting gibberellin biosynthesis, reducing internodal growth to give stouter stems, increasing root growth, causing early fruitset and increasing seedset in plants such as tomato and pepper. PBZ has also been shown to reduce frost sensitivity in plants. Moreover, paclobutrazol can be used as a chemical approach for reducing the risk of lodging in cereal crops. PBZ has been used by arborists to reduce shoot growth and shown to have additional positive effects on trees and shrubs. Among those are improved resistance to drought stress, darker green leaves, higher resistance against fungi and bacteria, and enhanced development of roots. Cambial growth, as well as shoot growth, has been shown to be reduced in some tree species.
GAI or Gibberellic-Acid Insensitive is a gene in Arabidopsis thaliana which is involved in regulation of plant growth. GAI represses the pathway of gibberellin-sensitive plant growth. It does this by way of its conserved DELLA motif.
Peptide signaling plays a significant role in various aspects of plant growth and development and specific receptors for various peptides have been identified as being membrane-localized receptor kinases, the largest family of receptor-like molecules in plants. Signaling peptides include members of the following protein families.
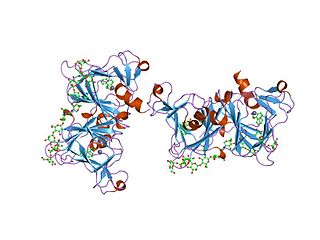
In molecular biology, the auxin binding protein family is a family of proteins which bind the plant hormone auxin. They are located in the lumen of the endoplasmic reticulum (ER). The primary structure of these proteins contains an N-terminal hydrophobic leader sequence of 30-40 amino acids, which could represent a signal for translocation of the protein to the ER. The mature protein comprises around 165 residues, and contains a number of potential N-glycosylation sites. In vitro transport studies have demonstrated co-translational glycosylation. Retention within the lumen of the ER correlates with an additional signal located at the C terminus, represented by the sequence Lys-Asp-Glu-Leu, known to be responsible for preventing secretion of proteins from the lumen of the ER in eukaryotic cells.
Ent-kaurene oxidase (EC 1.14.14.86, Formerly EC 1.14.13.78) is an enzyme with systematic name ent-kaur-16-ene,NADPH:oxygen oxidoreductase (hydroxylating). This enzyme catalyses the following chemical reaction
L-tryptophan—pyruvate aminotransferase is an enzyme with systematic name L-tryptophan:pyruvate aminotransferase. This enzyme catalyses the following chemical reaction
PIN proteins are integral membrane proteins in plants that transport the anionic form of the hormone auxin across membranes. The discovery of the initial member of the PIN gene family, PIN1, occurred through the identification of the pin-formed1 (pin1) mutation in Arabidopsis thaliana. This mutation led to a stem that lacked almost all organs, including leaves and flowers.

Ethylene (CH
2=CH
2) is an unsaturated hydrocarbon gas (alkene) acting as a naturally occurring plant hormone. It is the simplest alkene gas and is the first gas known to act as hormone. It acts at trace levels throughout the life of the plant by stimulating or regulating the ripening of fruit, the opening of flowers, the abscission (or shedding) of leaves and, in aquatic and semi-aquatic species, promoting the 'escape' from submergence by means of rapid elongation of stems or leaves. This escape response is particularly important in rice farming. Commercial fruit-ripening rooms use "catalytic generators" to make ethylene gas from a liquid supply of ethanol. Typically, a gassing level of 500 to 2,000 ppm is used, for 24 to 48 hours. Care must be taken to control carbon dioxide levels in ripening rooms when gassing, as high temperature ripening (20 °C; 68 °F) has been seen to produce CO2 levels of 10% in 24 hours.
Christoph Benning is a German–American plant biologist. He is an MSU Foundation Professor and University Distinguished Professor at Michigan State University. Benning's research into lipid metabolism in plants, algae and photosynthetic bacteria, led him to be named Editor-in-Chief of The Plant Journal in October 2008.
















