Antihypertensives are a class of drugs that are used to treat hypertension. Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke, heart failure, kidney failure and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, ACE inhibitors, angiotensin II receptor antagonists (ARBs), and beta blockers.
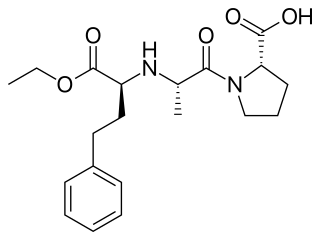
Enalapril, sold under the brand name Vasotec among others, is an ACE inhibitor medication used to treat high blood pressure, diabetic kidney disease, and heart failure. For heart failure, it is generally used with a diuretic, such as furosemide. It is given by mouth or by injection into a vein. Onset of effects are typically within an hour when taken by mouth and last for up to a day.

Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system.

Fluticasone/salmeterol, sold under the brand name Advair among others, is a fixed-dose combination medication containing fluticasone propionate, an inhaled corticosteroid; and salmeterol, a long-acting beta2‑adrenergic agonist. It is used in the management of asthma and chronic obstructive pulmonary disease (COPD). It is used by inhaling the medication into the lungs.
Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth.
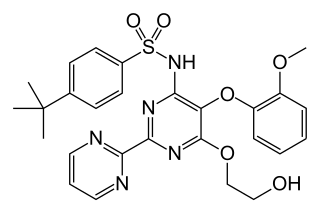
Bosentan, sold under the brand name Tracleer among others, is a dual endothelin receptor antagonist medication used in the treatment of pulmonary artery hypertension (PAH).
Ustekinumab, sold under the brand name Stelara among others, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.
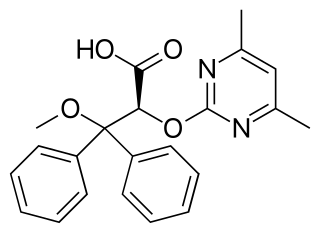
Ambrisentan, sold under the brand name Letairis among others, is a drug used for the treatment of pulmonary hypertension. It is an endothelin receptor antagonist.

Azilsartan, sold under the brand name Edarbi among others, is used for the treatment of hypertension. It is used as the prodrug azilsartan medoxomil, is an angiotensin II receptor antagonist, and was developed by Takeda.

Actelion Pharmaceuticals Ltd. is a pharmaceuticals and biotechnology company established in December 1997, headquartered in Allschwil near Basel, Switzerland.

Macitentan, sold under the brand name Opsumit, is an endothelin receptor antagonist developed by Actelion and approved for the treatment of pulmonary arterial hypertension (PAH). Macitentan is a dual endothelin receptor antagonist, meaning that it acts as an antagonist of two endothelin (ET) receptor subtypes, ETA and ETB. However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype.
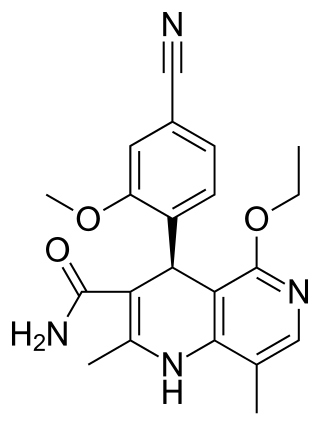
Finerenone, sold under the brand name Kerendia and Firialta, is a medication used to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adults with chronic kidney disease associated with type 2 diabetes. Finerenone is a non-steroidal mineralocorticoid receptor antagonist (MRA). It is taken orally.
Erdafitinib, sold under the brand name Balversa, is an anti-cancer medication. It is a small molecule inhibitor of fibroblast growth factor receptor (FGFR) used for the treatment of cancer. FGFRs are a subset of tyrosine kinases which are unregulated in some tumors and influence tumor cell differentiation, proliferation, angiogenesis, and cell survival. Astex Pharmaceuticals discovered the drug and licensed it to Janssen Pharmaceuticals for further development.

Daridorexant, sold under the brand name Quviviq, is an orexin antagonist medication which is used for the treatment of insomnia. Daridorexant is taken by mouth.
Ciltacabtagene autoleucel, sold under the brand name Carvykti, is an anti-cancer medication used to treat multiple myeloma. Ciltacabtagene autoleucel is a BCMA -directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using the recipient's own T-cells, which are collected and genetically modified, and infused back into the recipient.
Teclistamab, sold under the brand name Tecvayli, is a human bispecific monoclonal antibody used for the treatment of relapsed and refractory multiple myeloma. It is a bispecific antibody that targets the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA), which is expressed on the surface of malignant multiple myeloma B-lineage cells.

Sparsentan, sold under the brand name Filspari, is a medication used for the treatment of primary immunoglobulin A nephropathy. Sparsentan is an endothelin and angiotensin II receptor antagonist. It is taken by mouth.
Niraparib/abiraterone acetate, sold under the brand name Akeega, is a fixed-dose combination anti-cancer medication used for the treatment of prostate cancer. It contains niraparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, and abiraterone acetate, a CYP17 inhibitor.
Macitentan/tadalafil, sold under the brand name Opsynvi, is a fixed dose combination medication used for the treatment of pulmonary arterial hypertension. It contains macitentan, an endothelin receptor antagonist (ERA); and tadalafil, a phosphodiesterase 5 (PDE5) inhibitor.













