
A cell wall is a structural layer that surrounds some cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, and functions as a selective barrier. Another vital role of the cell wall is to help the cell withstand osmotic pressure and mechanical stress. While absent in many eukaryotes, including animals, cell walls are prevalent in other organisms such as fungi, algae and plants, and are commonly found in most prokaryotes, with the exception of mollicute bacteria.

Root pressure is the transverse osmotic pressure within the cells of a root system that causes sap to rise through a plant stem to the leaves.

In botany, a tendril is a specialized stem, leaf or petiole with a threadlike shape used by climbing plants for support and attachment, as well as cellular invasion by parasitic plants such as Cuscuta. There are many plants that have tendrils; including sweet peas, passionflower, grapes and the Chilean glory-flower. Tendrils respond to touch and to chemical factors by curling, twining, or adhering to suitable structures or hosts. Tendrils vary greatly in size from a few centimeters up to 27 inches for Nepenthes harryana The chestnut vine can have tendrils up to 20.5 inches in length. Normally there is only one simple or branched tendril at each node, but the aardvark cucumber can have as many as eight.
A tracheid is a long and tapered lignified cell in the xylem of vascular plants. It is a type of conductive cell called a tracheary element. Angiosperms use another type of conductive cell, called vessel elements, to transport water through the xylem. The main functions of tracheid cells are to transport water and inorganic salts, and to provide structural support for trees. There are often pits on the cell walls of tracheids, which allows for water flow between cells. Tracheids are dead at functional maturity and do not have a protoplast. The wood (softwood) of gymnosperms such as pines and other conifers is mainly composed of tracheids. Tracheids are also the main conductive cells in the primary xylem of ferns.

The endodermis is the innermost layer of cortex in land plants. It is a cylinder of compact living cells, the radial walls of which are impregnated with hydrophobic substances to restrict apoplastic flow of water to the inside. The endodermis is the boundary between the cortex and the stele.

Sap is a fluid transported in xylem cells or phloem sieve tube elements of a plant. These cells transport water and nutrients throughout the plant.

The Casparian strip is a band-like thickening in the center of the root endodermis of vascular plants. The composition of the region is mainly suberin, lignin and some structural proteins, which are capable of reducing the diffusive apoplastic flow of water and solutes into the stele and its width varies between species. The Casparian strip is impervious to water so can control the transportation of water and inorganic salts between the cortex and the vascular bundle, preventing water and inorganic salts from being transported to the stele through the apoplast, so that it must enter the cell membrane and move to the stele through the symplastic pathway, blocking the internal and external objects of the cell. The function of mass transportation are similar to that of animal tissues.. The development of the Casparian strip is regulated by transcription factors such as SHORT-ROOT (SHR), SCARECROW (SCR) and MYB36, as well as polypeptide hormone synthesised by midcolumn cells.

The symplast of a plant is the region enclosed by the cell membranes, within which water and solutes can diffuse freely. By contrast the apoplast is any fluid-filled space within the cell wall and extracellular space. Neighbouring cells are interconnected by microscopic channels known as plasmodesmata that traverse the cell walls. These channels, allow the flow of small molecules such as sugars, amino acids, and ions between cells. Larger molecules, including transcription factors and plant viruses, can also be transported through with the help of actin structures. The symplast allows direct cytoplasm-to-cytoplasm flow of water and other nutrients along concentration gradients. In particular, symplastic flow is used in the root systems to bring in nutrients from soil. Nutrient solutes move in this way through three skin layers of the roots: from cells of the epidermis, the outermost layer, through the cortex into the endodermis.
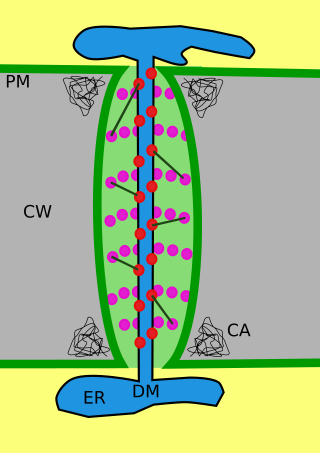
Plasmodesmata are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages, and species that have these structures include members of the Charophyceae, Charales, Coleochaetales and Phaeophyceae, as well as all embryophytes, better known as land plants. Unlike animal cells, almost every plant cell is surrounded by a polysaccharide cell wall. Neighbouring plant cells are therefore separated by a pair of cell walls and the intervening middle lamella, forming an extracellular domain known as the apoplast. Although cell walls are permeable to small soluble proteins and other solutes, plasmodesmata enable direct, regulated, symplastic transport of substances between cells. There are two forms of plasmodesmata: primary plasmodesmata, which are formed during cell division, and secondary plasmodesmata, which can form between mature cells.
The ascent of sap in the xylem tissue of plants is the upward movement of water and minerals from the root to the aerial parts of the plant. The conducting cells in xylem are typically non-living and include, in various groups of plants, vessel members and tracheids. Both of these cell types have thick, lignified secondary cell walls and are dead at maturity. Although several mechanisms have been proposed to explain how sap moves through the xylem, the cohesion-tension mechanism has the most support. Although cohesion-tension has received criticism due to the apparent existence of large negative pressures in some living plants, experimental and observational data favor this mechanism.
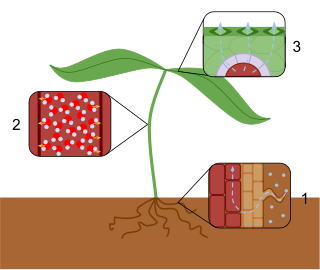
In plants, the transpiration stream is the uninterrupted stream of water and solutes which is taken up by the roots and transported via the xylem to the leaves where it evaporates into the air/apoplast-interface of the substomatal cavity. It is driven by capillary action and in some plants by root pressure. The main driving factor is the difference in water potential between the soil and the substomatal cavity caused by transpiration.
In biology, cell signaling is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes.
The pressure flow hypothesis, also known as the mass flow hypothesis, is the best-supported theory to explain the movement of sap through the phloem of plants. It was proposed by Ernst Münch, a German plant physiologist in 1930. Organic molecules such as sugars, amino acids, certain hormones, and messenger RNAs are known to be transported in the phloem through the cells called sieve tube elements. According to the hypothesis, high concentration of organic substances, particularly sugar, inside the phloem at a source such as a leaf, creates a diffusion gradient that draws water into the cells from the adjacent xylem. This creates turgor pressure, also called hydrostatic pressure, in the phloem. The hypothesis states that this is why movement of sap in the plant flows from the sugar producers (sources) to sugar absorbers (sinks).
Biotic stress is stress that occurs as a result of damage done to an organism by other living organisms, such as bacteria, viruses, fungi, parasites, beneficial and harmful insects, weeds, and cultivated or native plants. It is different from abiotic stress, which is the negative impact of non-living factors on the organisms such as temperature, sunlight, wind, salinity, flooding and drought. The types of biotic stresses imposed on an organism depend the climate where it lives as well as the species' ability to resist particular stresses. Biotic stress remains a broadly defined term and those who study it face many challenges, such as the greater difficulty in controlling biotic stresses in an experimental context compared to abiotic stress.

The Hartig net is the network of inward-growing hyphae, that extends into the plant host root, penetrating between plant cells in the root epidermis and cortex in ectomycorrhizal symbiosis. This network is the internal component of fungal morphology in ectomycorrhizal symbiotic structures formed with host plant roots, in addition to a hyphal mantle or sheath on the root surface, and extramatrical mycelium extending from the mantle into the surrounding soil. The Hartig net is the site of mutualistic resource exchange between the fungus and the host plant. Essential nutrients for plant growth are acquired from the soil by exploration and foraging of the extramatrical mycelium, then transported through the hyphal network across the mantle and into the Hartig net, where they are released by the fungi into the root apoplastic space for uptake by the plant. The hyphae in the Hartig net acquire sugars from the plant root, which are transported to the external mycelium to provide a carbon source to sustain fungal growth.
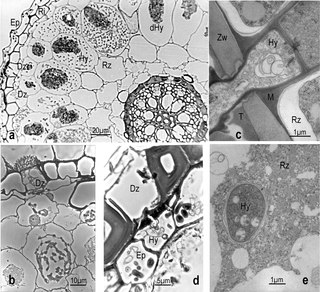
The exodermis is a physiological barrier that has a role in root function and protection. The exodermis is a membrane of variable permeability responsible for the radial flow of water, ions, and nutrients. It is the outer layer of a plant's cortex. The exodermis serves a double function as it can protect the root from invasion by foreign pathogens and ensures that the plant does not lose too much water through diffusion through the root system and can properly replenish its stores at an appropriate rate.

Phloem loading is the process of loading carbon into the phloem for transport to different 'sinks' in a plant. Sinks include metabolism, growth, storage, and other processes or organs that need carbon solutes to persist. It can be a passive process, relying on a pressure gradient to generate diffusion of solutes through the symplast, or an active process, requiring energy to create membrane-bound transporter proteins that move solutes through the apoplast against a gradient. Passive phloem loading transports solutes freely through plasmodesma in the symplast of the minor veins of leaves. Active transport occurs apoplastically and does not use plasmodesmata. An intermediate type of loading exists that uses symplastic transport but utilizes a size-exclusion mechanism to ensure diffusion is a one-way process between the mesophyll and phloem cells. This process is referred to as polymer-trapping, in which simple solutes such as sucrose are synthesized into larger molecules such as stachyose or raffinose in intermediary cells. The larger molecules cannot diffuse back to the mesophyll but can move into the phloem's sieve cells. Therefore, the synthesis of larger compounds uses energy and is thus 'active' but this strategy does not require specialized proteins and can still move symplastically.

Strigolactones are a group of chemical compounds produced by roots of plants. Due to their mechanism of action, these molecules have been classified as plant hormones or phytohormones. So far, strigolactones have been identified to be responsible for three different physiological processes: First, they promote the germination of parasitic organisms that grow in the host plant's roots, such as Strigalutea and other plants of the genus Striga. Second, strigolactones are fundamental for the recognition of the plant by symbiotic fungi, especially arbuscular mycorrhizal fungi, because they establish a mutualistic association with these plants, and provide phosphate and other soil nutrients. Third, strigolactones have been identified as branching inhibition hormones in plants; when present, these compounds prevent excess bud growing in stem terminals, stopping the branching mechanism in plants.
The acid-growth hypothesis is a theory that explains the expansion dynamics of cells and organs in plants. It was originally proposed by Achim Hager and Robert Cleland in 1971. They hypothesized that the naturally occurring plant hormone, auxin (indole-3-acetic acid, IAA), induces H+ proton extrusion into the apoplast. Such derived apoplastic acidification then activates a range of enzymatic reactions which modifies the extensibility of plant cell walls. Since its formulation in 1971, the hypothesis has stimulated much research and debate. Most debates have concerned the signalling role of auxin and the molecular nature of cell wall modification. The current version holds that auxin activates small auxin-up RNA (SAUR) proteins, which in turn regulate protein phosphatases that modulate proton-pump activity. Acid growth is responsible for short-term (seconds to minutes) variation in growth rate, but many other mechanisms influence longer-term growth.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.













