Related Research Articles
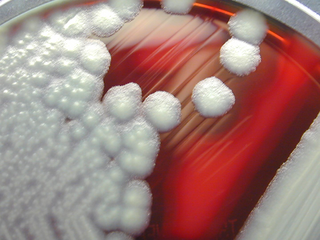
Bacillus cereus is a Gram-positive rod-shaped bacterium commonly found in soil, food, and marine sponges. The specific name, cereus, meaning "waxy" in Latin, refers to the appearance of colonies grown on blood agar. Some strains are harmful to humans and cause foodborne illness due to their spore-forming nature, while other strains can be beneficial as probiotics for animals, and even exhibit mutualism with certain plants. B. cereus bacteria may be anaerobes or facultative anaerobes, and like other members of the genus Bacillus, can produce protective endospores. They have a wide range of virulence factors, including phospholipase C, cereulide, sphingomyelinase, metalloproteases, and cytotoxin K, many of which are regulated via quorum sensing. B. cereus strains exhibit flagellar motility.
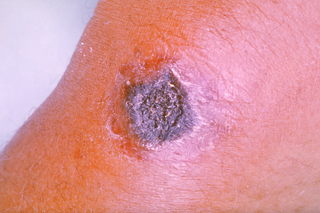
Anthrax is an infection caused by the bacterium Bacillus anthracis. It can occur in four forms: skin, lungs, intestinal, and injection. Symptom onset occurs between one day and more than two months after the infection is contracted. The skin form presents with a small blister with surrounding swelling that often turns into a painless ulcer with a black center. The inhalation form presents with fever, chest pain and shortness of breath. The intestinal form presents with diarrhea, abdominal pains, nausea and vomiting. The injection form presents with fever and an abscess at the site of drug injection.
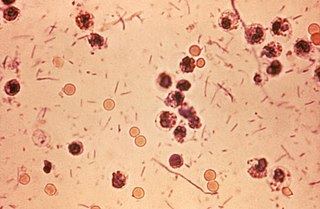
Shigella is a genus of bacteria that is Gram-negative, facultatively anaerobic, non–spore-forming, nonmotile, rod-shaped, and is genetically closely related to Escherichia. The genus is named after Kiyoshi Shiga, who discovered it in 1897.
Serial passage is the process of growing bacteria or a virus in iterations. For instance, a virus may be grown in one environment, and then a portion of that virus population can be removed and put into a new environment. This process is repeated with as many stages as desired, and then the final product is studied, often in comparison with the original virus.

The bacteria capsule is a large structure common to many bacteria. It is a polysaccharide layer that lies outside the cell envelope, and is thus deemed part of the outer envelope of a bacterial cell. It is a well-organized layer, not easily washed off, and it can be the cause of various diseases.
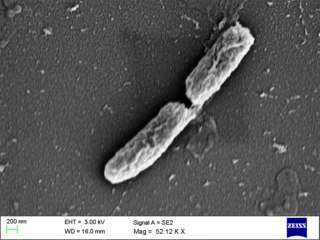
Shigella flexneri is a species of Gram-negative bacteria in the genus Shigella that can cause diarrhea in humans. Several different serogroups of Shigella are described; S. flexneri belongs to group B. S. flexneri infections can usually be treated with antibiotics, although some strains have become resistant. Less severe cases are not usually treated because they become more resistant in the future. Shigella are closely related to Escherichia coli, but can be differentiated from E.coli based on pathogenicity, physiology and serology.
Anthrax vaccines are vaccines to prevent the livestock and human disease anthrax, caused by the bacterium Bacillus anthracis.

Burkholderia pseudomallei is a Gram-negative, bipolar, aerobic, motile rod-shaped bacterium. It is a soil-dwelling bacterium endemic in tropical and subtropical regions worldwide, particularly in Thailand and northern Australia. It was reported in 2008 that there had been an expansion of the affected regions due to significant natural disasters, and it could be found in Southern China, Hong Kong, and countries in America. B. pseudomallei, amongst other pathogens, has been found in monkeys imported into the United States from Asia for laboratory use, posing a risk that the pathogen could be introduced into the country.
Dichelobacter nodosus, formerly Bacteroides nodosus, is a Gram-negative, obligate anaerobe of the family Cardiobacteriaceae. It has polar fimbriae and is the causative agent of ovine foot rot as well as interdigital dermatitis. It is the lone species in the genus Dichelobacter.
Burkholderia thailandensis is a nonfermenting motile, Gram-negative bacillus that occurs naturally in soil. It is closely related to Burkholderia pseudomallei, but unlike B. pseudomallei, it only rarely causes disease in humans or animals. The lethal inoculum is approximately 1000 times higher than for B. pseudomallei. It is usually distinguished from B. pseudomallei by its ability to assimilate arabinose. Other differences between these species include lipopolysaccharide composition, colony morphology, and differences in metabolism.
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen, but still keeping it viable. Attenuation takes an infectious agent and alters it so that it becomes harmless or less virulent. These vaccines contrast to those produced by "killing" the pathogen.

Rhodococcus equi is a Gram-positive coccobacillus bacterium. The organism is commonly found in dry and dusty soil and can be important for diseases of domesticated animals. The frequency of infection can reach near 60%. R. equi is an important pathogen causing pneumonia in foals. Since 2008, R. equi has been known to infect wild boar and domestic pigs. R. equi can infect humans. At-risk groups are immunocompromised people, such as HIV-AIDS patients or transplant recipients. Rhodococcus infection in these patients resemble clinical and pathological signs of pulmonary tuberculosis. It is facultative intracellular.
Segrosomes are protein complexes that ensure accurate segregation (partitioning) of plasmids or chromosomes during bacterial cell division.

Bacillus anthracis is a gram-positive and rod-shaped bacterium that causes anthrax, a deadly disease to livestock and, occasionally, to humans. It is the only permanent (obligate) pathogen within the genus Bacillus. Its infection is a type of zoonosis, as it is transmitted from animals to humans. It was discovered by a German physician Robert Koch in 1876, and became the first bacterium to be experimentally shown as a pathogen. The discovery was also the first scientific evidence for the germ theory of diseases.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxins. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.

Anthrax vaccine adsorbed, sold under the brand name Biothrax among others, is a vaccine intended to provide acquired immunity against Bacillus anthracis.
Reverse genetics is a method in molecular genetics that is used to help understand the function(s) of a gene by analysing the phenotypic effects caused by genetically engineering specific nucleic acid sequences within the gene. The process proceeds in the opposite direction to forward genetic screens of classical genetics. While forward genetics seeks to find the genetic basis of a phenotype or trait, reverse genetics seeks to find what phenotypes are controlled by particular genetic sequences.
The exosporium is the outer surface layer of mature spores. In plant spores it is also referred to as the exine. Some bacteria also produce endospores with an exosporium, of which the most commonly studied are Bacillus species, particularly Bacillus cereus and the anthrax-causing bacterium Bacillus anthracis. The exosporium is the portion of the spore that interacts with the environment or host organism, and may contain spore antigens. Exosporium proteins, such as Cot protein, are also discovered related to strains of B. anthracis and B.cereus. This Cot protein share similar sequences with other spore coat proteins, and their putative determinants are believed to include bxpC, lunA, exsA, etc.
Bacillus cereus biovar anthracis is a variant of the Bacillus cereus bacterium that has acquired plasmids similar to those of Bacillus anthracis. As a result, it is capable of causing anthrax. In 2016, it was added to the CDC's list of select agents and toxins.
Theresa Marie Koehler is an American microbiologist who is the Herbert L. and Margaret W. DuPont Distinguished Professor in Biomedical Sciences and Chair of the Department of Microbiology and Molecular Genetics at McGovern Medical School. She is known for her extensive research on anthrax and was elected Fellow of the American Association for the Advancement of Science in 2021.
References
- ↑ Rasko DA, Worsham PL, Abshire TG, Stanley ST, Bannan JD, Wilson MR, et al. (March 2011). "Bacillus anthracis comparative genome analysis in support of the Amerithrax investigation". Proceedings of the National Academy of Sciences of the United States of America. 108 (12): 5027–32. Bibcode:2011PNAS..108.5027R. doi: 10.1073/pnas.1016657108 . PMC 3064363 . PMID 21383169.
- ↑ Warrick J (2002-01-29). "One Anthrax Answer: Ames Strain Not From Iowa". The Washington Post.
- ↑ Fainaru S, Warrick J (2001-11-25). "Deadly Anthrax Strain Leaves a Muddy Trail". Washington Post.
- ↑ Coker PR, Smith KL, Fellows PF, Rybachuck G, Kousoulas KG, Hugh-Jones ME (March 2003). "Bacillus anthracis virulence in Guinea pigs vaccinated with anthrax vaccine adsorbed is linked to plasmid quantities and clonality". Journal of Clinical Microbiology. 41 (3): 1212–8. doi:10.1128/JCM.41.3.1212-1218.2003. PMC 150325 . PMID 12624053.
- ↑ Welkos SL, Vietri NJ, Gibbs PH (May 1993). "Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence". Microbial Pathogenesis. 14 (5): 381–8. doi:10.1006/mpat.1993.1037. PMID 8366815.
- ↑ Turnbull, Peter CB; Sirianni, Nicky M; LeBron, Carlos I; Samaan, Marian N; Sutton, Felicia N; Reyes, Anatalio E; Peruski, Leonard F (August 2004). "MICs of Selected Antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a Range of Clinical and Environmental Sources as Determined by the Etest". Journal of Clinical Microbiology. 42 (8): 3626–3634. doi:10.1128/jcm.42.8.3626-3634.2004. PMC 497625 . PMID 15297508.
- ↑ Heine, HS; Shadomy, SV; Boyer, AE; Chuvala, L; Riggins, R; Kerterson, A; Myrick, J; Craig, J; Candela, MG; Barr, JR; Hendricks, K; Bower, WA; Walke, H; Drusano, GL (September 2017). "Evaluation of Combination Drug Therapy for Treatment of Antibiotic-Resistant Inhalation Anthrax in a Murine Model". Antimicrobial Agents and Chemotherapy. 61 (9): e00788-17. doi:10.1128/AAC.00788-17. PMC 5571330 . PMID 28696235.
- ↑ Hendricks, Katherine A; Wright, Mary E; Shadomy, Sean V; Bradley, John S; Morrow, Meredith G; Pavia, Andy T; Rubinstein, Ethan; Holty, Jon-Erik C; Messonier, Nancy E; Smith, Theresa L; Pesik, Nicki; Treadwell, Tracee A; Bower, William A (February 2014). "Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults". Emerging Infectious Diseases. 20 (2): e130687. doi:10.3201/eid2002.130687. PMC 3901462 . PMID 24447897.
- ↑ "M45 Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd Edition". CLSI. Retrieved 14 June 2023.
- ↑ Steward J, Lever MS, Simpson AJ, Sefton AM, Brooks TJ (July 2004). "Post-exposure prophylaxis of systemic anthrax in mice and treatment with fluoroquinolones". The Journal of Antimicrobial Chemotherapy. 54 (1): 95–9. doi: 10.1093/jac/dkh276 . PMID 15163650.
- ↑ Chen Y, Succi J, Tenover FC, Koehler TM (February 2003). "Beta-lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain". Journal of Bacteriology. 185 (3): 823–30. doi:10.1128/JB.185.3.823-830.2003. PMC 142833 . PMID 12533457.
- ↑ "CDC - Anthrax Sterne, General Information - NCZVED". www.cdc.gov. Retrieved 2020-11-25.
- 1 2 Brossier F, Levy M, Mock M (February 2002). "Anthrax spores make an essential contribution to vaccine efficacy". Infection and Immunity. 70 (2): 661–4. doi: 10.1128/iai.70.2.661-664.2002 . PMC 127709 . PMID 11796596.
- ↑ Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM (November 2001). "Erratum to "Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin" [Vaccine 19 (2001) 3241–3247". Vaccine. 20 (3–4): 635. doi:10.1016/s0264-410x(01)00411-x.
- ↑ Van Ert MN, Easterday WR, Simonson TS, U'Ren JM, Pearson T, Kenefic LJ, et al. (January 2007). "Strain-specific single-nucleotide polymorphism assays for the Bacillus anthracis Ames strain". Journal of Clinical Microbiology. 45 (1): 47–53. doi:10.1128/JCM.01233-06. PMC 1828967 . PMID 17093023.
- ↑ Rasko DA, Worsham PL, Abshire TG, Stanley ST, Bannan JD, Wilson MR, et al. (March 2011). "Bacillus anthracis comparative genome analysis in support of the Amerithrax investigation". Proceedings of the National Academy of Sciences of the United States of America. 108 (12): 5027–32. Bibcode:2011PNAS..108.5027R. doi: 10.1073/pnas.1016657108 . PMC 3064363 . PMID 21383169.