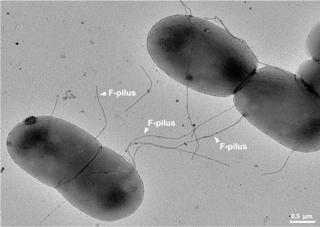
Bacterial conjugation is the transfer of genetic material between bacterial cells by direct cell-to-cell contact or by a bridge-like connection between two cells. This takes place through a pilus. It is a parasexual mode of reproduction in bacteria.

In molecular biology and genetics, transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of exogenous genetic material from its surroundings through the cell membrane(s). For transformation to take place, the recipient bacterium must be in a state of competence, which might occur in nature as a time-limited response to environmental conditions such as starvation and cell density, and may also be induced in a laboratory.
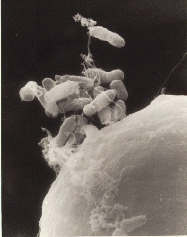
Agrobacterium tumefaciens is the causal agent of crown gall disease in over 140 species of eudicots. It is a rod-shaped, Gram-negative soil bacterium. Symptoms are caused by the insertion of a small segment of DNA, from a plasmid into the plant cell, which is incorporated at a semi-random location into the plant genome. Plant genomes can be engineered by use of Agrobacterium for the delivery of sequences hosted in T-DNA binary vectors.
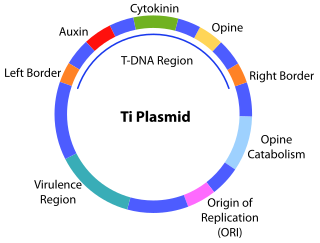
The transfer DNA is the transferred DNA of the tumor-inducing (Ti) plasmid of some species of bacteria such as Agrobacterium tumefaciens and Agrobacterium rhizogenes . The T-DNA is transferred from bacterium into the host plant's nuclear DNA genome. The capability of this specialized tumor-inducing (Ti) plasmid is attributed to two essential regions required for DNA transfer to the host cell. The T-DNA is bordered by 25-base-pair repeats on each end. Transfer is initiated at the right border and terminated at the left border and requires the vir genes of the Ti plasmid.

In genetic engineering, a gene gun or biolistic particle delivery system is a device used to deliver exogenous DNA (transgenes), RNA, or protein to cells. By coating particles of a heavy metal with a gene of interest and firing these micro-projectiles into cells using mechanical force, an integration of desired genetic information can be introduced into desired cells. The technique involved with such micro-projectile delivery of DNA is often referred to as biolistics, short for "biological ballistics".

A tumour inducing (Ti) plasmid is a plasmid found in pathogenic species of Agrobacterium, including A. tumefaciens, A. rhizogenes, A. rubi and A. vitis.

Rhizobium rhizogenes is a Gram-negative soil bacterium that produces hairy root disease in dicotyledonous plants. R. rhizogenes induces the formation of proliferative multiple-branched adventitious roots at the site of infection, so-called 'hairy roots'. It also induces galls.
Alphaproteobacteria is a class of bacteria in the phylum Pseudomonadota. The Magnetococcales and Mariprofundales are considered basal or sister to the Alphaproteobacteria. The Alphaproteobacteria are highly diverse and possess few commonalities, but nevertheless share a common ancestor. Like all Proteobacteria, its members are gram-negative, although some of its intracellular parasitic members lack peptidoglycan and are consequently gram variable.

Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Gene delivery must reach the genome of the host cell to induce gene expression. Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors.

Jozef Stefaan "Jeff", Baron Schell was a Belgian molecular biologist.
Plant transformation vectors are plasmids that have been specifically designed to facilitate the generation of transgenic plants. The most commonly used plant transformation vectors are T-DNA binary vectors and are often replicated in both E. coli, a common lab bacterium, and Agrobacterium tumefaciens, a plant-virulent bacterium used to insert the recombinant DNA into plants.
A transfer DNA (T-DNA) binary system is a pair of plasmids consisting of a T-DNA binary vector and a virhelper plasmid. The two plasmids are used together to produce genetically modified plants. They are artificial vectors that have been derived from the naturally occurring Ti plasmid found in bacterial species of the genus Agrobacterium, such as A. tumefaciens. The binary vector is a shuttle vector, so-called because it is able to replicate in multiple hosts.
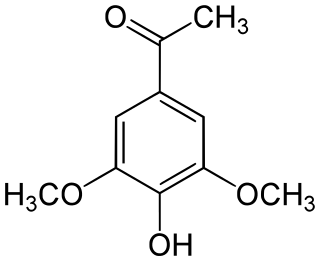
Acetosyringone is a phenolic natural product and a chemical compound related to acetophenone and 2,6-dimethoxyphenol. It was first described in relation to lignan/phenylpropanoid-type phytochemicals, with isolation from a variety of plant sources, in particular, in relation to wounding and other physiologic changes.
αr7 is a family of bacterial small non-coding RNAs with representatives in a broad group of Alphaproteobacterial species from the order Hyphomicrobiales. The first member of this family was found in a Sinorhizobium meliloti 1021 locus located in the chromosome (C). Further homology and structure conservation analysis identified full-length homologs in several nitrogen-fixing symbiotic rhizobia, in the plant pathogens belonging to Agrobacterium species as well as in a broad spectrum of Brucella species. αr7 RNA species are 134-159 nucleotides (nt) long and share a well defined common secondary structure. αr7 transcripts can be catalogued as trans-acting sRNAs expressed from well-defined promoter regions of independent transcription units within intergenic regions (IGRs) of the Alphaproteobacterial genomes.
αr15 is a family of bacterial small non-coding RNAs with representatives in a broad group of α-proteobacteria from the order Rhizobiales. The first members of this family were found tandemly arranged in the same intergenic region (IGR) of the Sinorhizobium meliloti 1021 chromosome (C). Further homology and structure conservation analysis have identified full-length Smr15C1 and Smr15C2 homologs in several nitrogen-fixing symbiotic rhizobia, in the plant pathogens belonging to Agrobacterium species as well as in a broad spectrum of Brucella species. The Smr15C1 and Smr15C2 homologs are also encoded in tandem within the same IGR region of Rhizobium and Agrobacterium species, whereas in Brucella species the αr15C loci are spread in the IGRs of Chromosome I. Moreover, this analysis also identified a third αr15 loci in extrachromosomal replicons of the mentioned nitrogen-fixing α-proteobacteria and in the Chromosome II of Brucella species. αr15 RNA species are 99-121 nt long and share a well defined common secondary structure consisting of three stem loops. The transcripts of the αr15 family can be catalogued as trans-acting sRNAs encoded by independent transcription units with recognizable promoter and transcription termination signatures within intergenic regions (IGRs) of the α-proteobacterial genomes.

Genetic engineering techniques allow the modification of animal and plant genomes. Techniques have been devised to insert, delete, and modify DNA at multiple levels, ranging from a specific base pair in a specific gene to entire genes. There are a number of steps that are followed before a genetically modified organism (GMO) is created. Genetic engineers must first choose what gene they wish to insert, modify, or delete. The gene must then be isolated and incorporated, along with other genetic elements, into a suitable vector. This vector is then used to insert the gene into the host genome, creating a transgenic or edited organism.
EHA101 was one of the first and most widely used Agrobacterium helper plasmid for plant gene transfer. Created in 1985 in the laboratory of Mary-Dell Chilton at Washington University in St. Louis, it was named after the graduate student who constructed it. The EH stands for "Elizabeth Hood" and A for "Agrobacterium". The EHA101 helper strain is a derivative of A281, the hypervirulent A. tumefaciens strain that causes large, fast-growing tumors on solanaceous plants. This strain is used for moving genes of interest into many hundreds of species of plants all over the world.
Allorhizobium vitis is a plant pathogen that infects grapevines. The species is best known for causing a tumor known as crown gall disease. One of the virulent strains, A. vitis S4, is responsible both for crown gall on grapevines and for inducing a hypersensitive response in other plant species. Grapevines that have been affected by crown gall disease produce fewer grapes than unaffected plants. Though not all strains of A. vitis are tumorigenic, most strains can damage plant hosts.
Agrobacterium radiobacter is the type species of the genus Agrobacterium. It was formerly incorrectly synonymized with Agrobacterium tumefaciens. Unlike other members of its genus, it does not harbor a tumor-inducing (Ti) plasmid, and is hence not pathogenic to plants. This species is widely found in soil, in plant rhizospheres, and in human clinical specimens.
The root inducing (Ri) -plasmid of Rhizobium rhizogenes is a plasmid capable of undergoing horizontal gene transfer of its transfer DNA (T-DNA), upon contact with a plant host. The T-DNA of the Ri-plasmid affects the plant host in such a way, that gene expression is altered, especially in regard to phytohormonal balances, metabolism and certain phenotypical characteristics.












