US20230137258A1 - Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element - Google Patents
Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element Download PDFInfo
- Publication number
- US20230137258A1 US20230137258A1 US17/850,832 US202217850832A US2023137258A1 US 20230137258 A1 US20230137258 A1 US 20230137258A1 US 202217850832 A US202217850832 A US 202217850832A US 2023137258 A1 US2023137258 A1 US 2023137258A1
- Authority
- US
- United States
- Prior art keywords
- potential
- electrode
- polymer film
- conducting polymer
- tissue
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/20—Applying electric currents by contact electrodes continuous direct currents
- A61N1/30—Apparatus for iontophoresis, i.e. transfer of media in ionic state by an electromotoric force into the body, or cataphoresis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/05—Detecting, measuring or recording for diagnosis by means of electric currents or magnetic fields; Measuring using microwaves or radio waves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/14546—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue for measuring analytes not otherwise provided for, e.g. ions, cytochromes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/1468—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue using chemical or electrochemical methods, e.g. by polarographic means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M5/00—Devices for bringing media into the body in a subcutaneous, intra-vascular or intramuscular way; Accessories therefor, e.g. filling or cleaning devices, arm-rests
- A61M5/14—Infusion devices, e.g. infusing by gravity; Blood infusion; Accessories therefor
- A61M5/168—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body
- A61M5/172—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body electrical or electronic
- A61M5/1723—Means for controlling media flow to the body or for metering media to the body, e.g. drip meters, counters ; Monitoring media flow to the body electrical or electronic using feedback of body parameters, e.g. blood-sugar, pressure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B2562/00—Details of sensors; Constructional details of sensor housings or probes; Accessories for sensors
- A61B2562/12—Manufacturing methods specially adapted for producing sensors for in-vivo measurements
- A61B2562/125—Manufacturing methods specially adapted for producing sensors for in-vivo measurements characterised by the manufacture of electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Definitions
- the present invention generally relates to electrochemical sensors.
- Electrochemical sensor devices have witnessed increased development activity in recent years, driven primarily by the challenge of in vivo glucose sensing. However, to date, these devices are manufactured using labor- and time-consuming physical assembly processes, especially to impart their selective chemical sensing capabilities. In this vein, such devices have not leveraged high-volume automated manufacturing processes to fabricate electrochemical sensors with high yield and uniformity.
- the poly(vbpy)Re(CO)3 Cl or poly[(vbpy)Re(CO)3 (MeCN)]+adsorbed onto the electrode acts as a catalyst for CO2 reduction to CO.
- Stability and the reactivity of the polymer film may be increased by co-reductive electropolymerization of either of the aforesaid Re complexes with [(bpy)2 Ru(vpy)2 2+(bis(4-vinyl-pyridine)bis(2,2′-bipyridine) ruthenium (II)) (vpy is 4-vinylpyridine).
- a bilayer film assembly consisting of poly-[(bpy)2 Ru(vpy)2 ]2+as an inner film and the aforementioned copolymer of Ru/Re yields even greater reactivity to CO2; a method of forming a polymeric film on an electrode; and a method of reducing CO2 to CO using an electrode having a polymeric film adsorbed to its surface.
- Yacynych, U.S. Pat. No. 5,540,828 for a Method for making electrochemical sensors and biosensors having a polymer modified surface discloses a method for making a sensing element for use in a sensor or biosensor that amperometrically measures the concentration of an analyte in a liquid, includes the following sequential steps: a) obtaining an electrode; b) immersing the electrode in a solution of monomer that is capable of being electropolymerized into an electrically insulating polymer; c) flowing an electric current from a cathode through the solution to the electrode at a voltage and amperage sufficient to cause the monomer to polymerize on the surface of the electrode, thereby yielding an electrode coated with an adherent layer of electrically insulating polymer; and e) impregnating the polymeric coating on the surface with a sensing agent that is capable, when contacted by a specific analyte in a chemical or biological liquid, of generating an electroactive molecule that can be detected
- a preferred biosensor includes an electrode having enzyme deposited thereon together with a layer of electropolymerized polymer intermingled with the enzyme; a crosslinked silane film is applied over the polymer layer, and a final coating of polyurethane is formed over the film.
- the enzyme is electrodeposited using an aqueous enzyme solution containing a nonionic surfactant at a concentration level preferably in excess of the critical micelle concentration of the surfactant.
- the polymer layer is preferably polyphenol, while the silane film is crosslinked (3-aminopropyl) trimethoxysilane.
- the preferred biosensors have greatly enhanced selectivity stabilities.
- Haesik et al. U.S. Pat. No. 6,413,396 for an Enzyme electrode sensor and manufacturing method thereof discloses an enzyme electrode sensor and a fabricating method thereof, and more particularly, an enzyme electrode sensor which is a biosensor using electrochemical measurement and a manufacturing method thereof.
- the sensor includes an electrode, a first nonconducting polymer layer formed by electropolymerization outside the electrode wherein enzyme is immobilized in the nonconducting polymer layer, a second nonconducting polymer layer in which enzyme is not immobilized, the second nonconducting layer formed by electropolymerization outside the first nonconducting polymer layer, and an outer layer formed outside the second nonconducting layer.
- the sensor selectivity is improved as the interference of organic materials is inhibited, and the interference of acetaminophen causing the major problem with a glucose sensor is controlled effectively by the sensor.
- Tissue-penetrating electrochemical sensors represent a promising avenue towards the minimally-invasive quantification of a number of relevant analytes in the physiological fluid, such as interstitial fluid, blood, serum, and plasma.
- membranes comprising of one or more polymers with a biorecognition element dispersed therein are cast in a manual fashion, keeping costs high and sensor-to-sensor uniformity low.
- tissue can refer to, but is not limited to, skin, organs, and cells.
- the technology disclosed herein addresses the above challenges by disclosing a method for the automated and parallelized co-deposition of a conducting polymer and biorecognition element at one or more working electrodes of a tissue-penetrating electrochemical sensor.
- the present invention provides for a method to impart the ability to selectively quantify chemical/biochemical analytes occupying physiological fluids via an automated process that allow the precise and spatially-defined simultaneous deposition of a thin-film of polymer containing an immobilized biorecognition element dispersed therein.
- the technology described herein also involves a method for automated, volume-scale functionalization of a tissue-penetrating electrochemical sensor to yield selective chemical or biochemical recognition capability in vivo.
- the present invention delineates a method for the simultaneous co-deposition of both an entrapment polymer and an immobilized biorecognition element onto at least one working electrode using an electro-deposition process for skin- and tissue-penetrating electrochemical sensor functionalization that is highly amenable to high-throughput, automated, parallelized, and highly reproducible manufacturing methods that do away with the need for a human operator during the sensor functionalization process.
- electro-deposition process for skin- and tissue-penetrating electrochemical sensor functionalization that is highly amenable to high-throughput, automated, parallelized, and highly reproducible manufacturing methods that do away with the need for a human operator during the sensor functionalization process.
- conventional manual processes associated with sensor functionalization to impart a selective biorecognition capability is becoming increasingly inaccessible using a human operator and the need remains for a sensor functionalization strategy that can be exploited along conventional precision manufacturing lines, such as those used for the medical device, MEMS, and semiconductor industries.
- tissue-penetrating electrochemical sensor device for the quantification of a chemical or biochemical entity in a physiological fluid.
- the device comprises a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode, and a conducting polymer film comprising an organic electroactive monomer precursor and at least one biorecognition element.
- the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the conducting polymer film.
- the conducting polymer film is produced by immersing the tissue-penetrating electrochemical sensor in a solution containing the at least one biomolecular recognition element and the organic electroactive monomer precursor which is dissolved in the solution, and wherein application of an oxidizing potential or a reducing potential at the spatially-defined working electrode causes the co-electrodeposition of the conducting polymer film.
- Another aspect of the present invention is a method for the fabrication of a tissue-penetrating electrochemical sensor for the quantification of a chemical or biochemical entity in a physiological fluid.
- the method includes immersing a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode in a solution comprising at least one biomolecular recognition element and an organic electroactive monomer precursor dissolved in the solution.
- the method also includes applying of an oxidizing potential or a reducing potential at the spatially-defined working electrode, causing a co-electrodeposition of a polymer film from the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the polymer film.
- the organic electroactive monomer precursor is preferably selected for at least one of its perm-selective properties following conversion to a thin-film of polymer, its charge rejection properties following conversion to a thin-film of polymer, its anti-biofouling properties following conversion to a thin-film of polymer, its porosity following conversion to a thin-film of polymer, its diffusion-limiting nature following conversion to a thin-film of polymer, or its self-limited growth during conversion to a thin-film of polymer.
- the biomolecular recognition element comprises at least one of an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- the tissue-penetrating electrochemical sensor further comprises at least a reference electrode or a counter electrode.
- the oxidizing potential or reducing potential is either a fixed potential or a time-varying potential.
- the oxidizing potential or reducing potential is applied using one of an amperometric technique, a voltammetric technique, a conductometric technique, or a coulometric technique.
- the oxidizing potential or the reducing potential is selected to result in the formation of a precise thickness of the conducting polymer film.
- the oxidizing potential or the reducing potential is applied for a specified time duration to result in the formation of a precise thickness of the conducting polymer film.
- the oxidizing potential or the reducing potential is selected to pass a specified amount of charge through at least one of the working electrode to result in the formation of a precise thickness of the conducting polymer film.
- FIG. 1 is an optical micrograph of an unfunctionalized working electrode.
- FIG. 2 is an optical micrograph of the working electrode shown in FIG. 1 following functionalization.
- FIG. 3 is an electron micrograph delineating the surface morphology of the functionalized working electrode provided in FIG. 2 .
- FIG. 4 is a block/process flow diagram illustrating the major constituents involved in the functionalization of a tissue- or skin-penetrating electrochemical sensor to facilitate chemical or biochemical quantification of various analytes in physiological fluids.
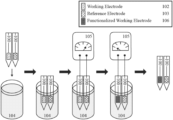
- FIG. 5 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating three-electrode electrochemical sensor.
- FIG. 6 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor.
- FIG. 7 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a multi-component tissue-penetrating three-electrode electrochemical sensor, whereby each electrode is located within an isolated tissue-penetration electrochemical sensor.
- FIG. 8 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor.
- FIG. 9 is a schematic representation delineating the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating electrochemical sensor.
- tissue-penetrating electrochemical sensor device for the quantification of a chemical or biochemical entity in a physiological fluid.
- the device preferably comprises a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode, and a conducting polymer film comprising an organic electroactive monomer precursor and at least one biorecognition element.
- the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the conducting polymer film.
- the conducting polymer film is produced by immersing the tissue-penetrating electrochemical sensor in a solution containing the at least one biomolecular recognition element and the organic electroactive monomer precursor which is dissolved in the solution, and wherein application of an oxidizing potential or a reducing potential at the spatially-defined working electrode causes the co-electrodeposition of the conducting polymer film.
- Another embodiment is a method for the fabrication of a tissue-penetrating electrochemical sensor for the quantification of a chemical or biochemical entity in a physiological fluid.
- the method includes immersing a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode in a solution comprising at least one biomolecular recognition element and an organic electroactive monomer precursor dissolved in the solution.
- the method also includes applying of an oxidizing potential or a reducing potential at the spatially-defined working electrode, causing a co-electrodeposition of a polymer film from the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the polymer film.
- the method provides for reliably dispensing a reagent containing at least one biorecognition at the micron-scale, as required to functionalize microelectrodes and microelectrode arrays.
- the method provides the formation of a polymeric film containing a biorecognition element by means of electro-polymerization enables the precise control of the thickness of the layer as well as the formation of a very thin layer.
- electro-polymerization utilized in the present invention is very effective for the reproducible formation of a layer as well as the simultaneous realization of co-deposition of both the polymer and the biorecognition element.
- Precisely-defined thin-films whose thickness and porosity can be precisely controlled by the suitable application of voltage, current, or charge that is passed through the electrode, also embody the advantage that the approach requires minimal quantity of reagent, thereby contributing to a reduction in cost to manufacture.
- the technology disclosed herein discloses a method for the automated and parallelized co-deposition of a conducting polymer and biorecognition element at one or more working electrodes of a tissue-penetrating electrochemical sensor.
- the method involves the immersion of the electrochemical sensor in a solution of an organic electroactive monomeric precursor (to the conducting polymer) and biorecognition element (enzyme, antibody, etc.) and applying a fixed- or time-varying potential at one or more of the working electrodes to instigate co-electrodeposition. This encourages the electro-polymerization of a conducting polymer film, synthesized from the monomeric precursor, onto the working electrode surface.
- the biorecognition element is also entrapped, in a spatially uniform fashion, in the polymer film and can, optionally, serve as the counter-ion or dopant during the electro-polymerization process through suitable choice of a biorecognition element with appropriate isoelectric point and solution pH.
- the thickness and porosity of the polymeric film is adjusted via modification of the applied potential, current, or charge passed through the electrochemical cell during the co-electrodeposition process. This has wide-ranging implications pertaining to the sensitivity, selectivity, stability, and response time of the as-synthesized tissue-penetrating electrochemical sensor.
- the disclosed technique facilitates spatially-defined deposition of precisely-controlled thin-films on one or more working electrode surfaces and allows the sensor designer to synthesize entrapment membranes to achieve certain utilitarian features such as co-circulating electroactive interference rejection (via perm- or charge-selectivity), increased linearity, extended lifetime, minimal signal drift, or reduced tendency to undergo biofouling when used in vivo.
- Simple and reproducible formations of chemically- and biochemically-selective polymer films can be formed with the disclosed method, which obviates the need for multiple biorecognition element casting and polymer casting steps by allowing for the simultaneous co-deposition of a thin-film containing both an entrapping polymer and biorecognition element.
- the as-disclosed sensor functionalization strategy enables parallelized sensor functionalization without human intervention and is amenable to implementation along assembly lines or semiconductor fabrication processes.
- the voltage/current. source is preferably a CH INSTRUMENTS Model 10000 Series Multi-Potentiostat, Metrohm Autolab PGSTAT Series Potentiostat Galvanostat, or Palmsens EmStat Potentiostat.
- the voltages utilized depend on the film, and generally range from ⁇ 0.2 volts to +1.0 volts.
- the current transduced ranges from 10 picoAmperes (pA) to 100 microAmperes ( ⁇ A), and is dependent on whether a singular electrode is electro-deposited or if multiple electrodes are electro-deposited as a group.
- the working electrode is preferably a CH INSTRUMENTS CHI101 2 mm-diameter gold disc microelectrode, or a METROHM MF-2150 100 ⁇ m-diameter platinum disc microelectrode.
- the tissue-penetrating electrochemical sensor is preferably a subcutaneous, percutaneous, transdermal, or intradermal sensor in which an electrical stimulus is applied to encourage a redox reaction and in which a voltage, current, charge, resistance, or impedance property is measured to infer the concentration of a particular chemical/biochemical analyte of interest present in the physiological fluid compartment in which the sensor is located.
- the sensor also comprises at least one working electrode (defined below) and at least one of a counter electrode and a reference electrode.
- the working electrode is preferably a spatially-defined electrode whereby the electrochemical sensing operation is to occur; functionalized with a polymer and biorecognition element to impart the selective ability to transduce the chemical/biochemical of interest into an electrical signal.
- a defined voltage, current, or amount of charge is applied as a stimulus to the working electrode to instigate a redox reaction and a voltage, current, charge, resistance, or impedance response is measured to infer the concentration of a particular chemical/biochemical analyte of interest.
- the organic electroactive monomer precursor is preferably an organic monomer that is either oxidized or reduced by the application of a suitable voltage, current, or amount of charge on a working electrode surface and concomitantly electro-deposited/electro-polymerized on the working electrode surface from a solution in which it is dissolved.
- the biorecognition element is preferably an organic, inorganic, or biological compound that selectively binds, catalyzes, or sieves a particular chemical/biochemical analyte of interest and, in doing so, changes its electrical properties (voltage, current, charge, resistance, or impedance) or produces an electroactive product (H 2 O 2 , NADH, etc.).
- a biorecognition element include, but are not limited to, an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- the conducting polymer is preferably synthesized from the organic electroactive monomer precursor through the process of electro-polymerization.
- the conducting polymer serves to physically entrap the biorecognition element in its highly porous matrix, thereby forming a thin-film with selective sensing capabilities.
- the biorecognition element can serve as the dopant or counter-ion through the appropriate selection of the biorecognition element's isoelectric point and solution pH in which the organic electroactive monomer precursor and biorecognition element are dissolved.
- the polymer can also be selected to exhibit perm-selective, charge-selective, size-selective, or anti-biofouling properties.
- the polymer film thickness preferably ranges from 5 nanometers (“nm”) to 50 nm.
- the thickness is primarily governed by the voltage applied, the time that the voltage is applied, the amount of the monomeric precursor in the solution, and the geometry of the electrode.
- the porosity of the film and the perm-selectivity of the film are also adjusted by these factors.
- the voltage or current source is either a power supply, potentiostat, galvanostat, function generator, or any source of electrical voltage or current.
- the voltage or current source is operated to apply a particular voltage, current, or amount of charge.
- the voltage or current source is terminated once a certain amount of time has transpired or charge has passed through the working electrode.
- the voltages utilized depend on the film and generally range from ⁇ 0.2 volts to +1.0 volts.
- the current transduced ranges from 10 picoAmperes (pA) to 100 microAmperes ( ⁇ A), and is dependent on whether a singular electrode is electro-deposited or if multiple electrodes are electro-deposited as a group.
- the solution composition comprises the organic electroactive monomeric precursor dissolved in H 2 0 and optionally salt(s) from the group consisting of NaCl, KCl, MgSO4, CaCl2, Na2HPO4, NaH2PO4, K2HPO4, and KH2PO4.
- the organic monomeric precursor is preferably one or more of, but not limited to, the following: aniline, pyrrole, acetylene, phenylene, phenylene vinylene, phenylene diamine, thiophene, 3,4-ethylenedioxythiophene, aminophenylboronic acid.
- the method steps of the invention include the following: 1) Immersion of a tissue-penetrating electrochemical sensor in a solution of at least one of a biorecognition element and an organic electroactive monomer precursor.
- a tissue-penetrating electrochemical sensor 100 containing, in this example, exactly one counter electrode 101 , one working electrode 102 , and one reference electrode 103 is immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a tissue-penetrating electrochemical sensor 100 containing, in this example, exactly one working electrode 102 and one reference electrode 103 is immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to counter electrode 101 and at least one of 102 and 103 ; a constant or time-varying potential is applied to counter electrode 101 to pass a controlled and pre-determined amount of current or charge through counter electrode 101 .
- organic electroactive monomer precursor an organic electroactive monomer precursor.
- the organic monomer is either oxidized or reduced by the application of a suitable voltage, current, or amount of charge on a working electrode surface and concomitantly electro-deposited/electro-polymerized on the working electrode surface from a solution in which it is dissolved.
- Biorecognition element An organic, inorganic, or biological compound that selectively binds, catalyzes, or sieves a particular chemical/biochemical analyte of interest and, in doing so, changes its electrical properties (voltage, current, charge, resistance, or impedance) or produces an electroactive product (H 2 O 2 , NADH, etc.).
- a biorecognition element include, but are not limited to, an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- the outputs of the invention are as follows: Functionalized working electrode.
- One or more working electrodes are coated with a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer 110 , which serves to entrap the biomolecular recognition element to form a co-deposited, highly porous polymer-biorecognition element matrix 109 .
- This processes functionalizes the working electrode 102 of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- FIG. 1 is an optical micrograph of an unfunctionalized working electrode.
- FIG. 2 is an optical micrograph of the working electrode shown in FIG. 1 following functionalization. The presence of a thin-film of co-electrodeposited polymer and enzyme can be observed from the image.
- FIG. 3 is an electron micrograph delineating the surface morphology of the functionalized working electrode provided in FIG. 2 .
- FIG. 4 is a block/process flow diagram illustrating the major constituents involved in the functionalization of a tissue- or skin-penetrating electrochemical sensor to facilitate chemical or biochemical quantification of various analytes in physiological fluids.
- FIG. 5 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating three-electrode electrochemical sensor.
- a tissue-penetrating electrochemical sensor 100 containing, in this example, exactly one counter electrode 101 , one working electrode 102 , and one reference electrode 103 is immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to 102 and at least one of 101 and 103 ; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102 .
- This in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein.
- This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- FIG. 6 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor.
- a tissue-penetrating electrochemical sensor 100 containing, in this example, exactly one working electrode 102 and one reference electrode 103 is immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to 102 and 103 ; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102 .
- This in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein.
- This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- FIG. 7 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a multi-component tissue-penetrating three-electrode electrochemical sensor, whereby each electrode is located within an isolated tissue-penetration electrochemical sensor.
- three tissue-penetrating electrochemical sensors 100 containing, in this example, exactly one counter electrode 101 , one working electrode 102 , and one reference electrode 103 are immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to 102 and at least one of 101 and 103 ; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102 .
- This in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein.
- This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- FIG. 8 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor.
- two tissue-penetrating electrochemical sensors 100 containing, in this example, exactly one working electrode 102 and one reference electrode 103 are immersed in a solution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to working electrode 102 and reference electrode 103 ; a constant or time-varying potential is applied to working electrode 102 to pass a controlled and pre-determined amount of current or charge through working electrode 102 .
- This in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein.
- This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- FIG. 9 is a schematic representation delineating the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode 102 of a tissue-penetrating electrochemical sensor.
- the working electrode 102 in this example is immersed in a solution 104 comprising at least one of a biomolecular recognition element ( 107 in inset) and at least one of an organic electroactive monomer precursor ( 108 in inset) dissolved in said solution.
- a voltage or current source 105 is connected, in an ohmic fashion, to working electrode 102 ; a constant or time-varying potential is applied to working electrode 102 to pass a controlled and pre-determined amount of current or charge through working electrode 102 .
- This instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer ( 110 in inset), which serves to entrap the biomolecular recognition element to form a co-deposited, highly porous polymer-biorecognition element matrix 109 .
- This processes functionalizes the working electrode 102 of the tissue-penetrating electrochemical sensor 100 and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid.
- Windmiller U.S. Provisional Patent Application No. 62/233,323, filed on Sep. 26, 2015, for a Methods And Devices For Embedding Sensors In Container Labels and Packaging is hereby incorporated by reference in its entirety.
- Windmiller et al. U.S. Provisional Patent Application No. 62/233,324, filed on Sep. 26, 2015, for a Method And System For Manufacturing Microneedles With Electrodes is hereby incorporated by reference in its entirety.
- Windmiller et al. U.S. Provisional Patent Application No. 62/247,732, filed on Oct. 28, 2015, for a Method And System For Three Dimensional Printing Of Microneedles And Microneedle Arrays For Transdermal Biosensing And Drug Delivery is hereby incorporated by reference in its entirety.
- Windmiller et al. U.S. Provisional Patent Application No. 62/247,730, filed on Oct. 28, 2015, for a Sensors For Wearable Devices is hereby incorporated by reference in its entirety.
- Windmiller U.S. Provisional Patent Application No. 62/307,444, filed on Mar. 12, 2016, for a Method And Apparatus For Determining Exhaled Carbon Loss is hereby incorporated by reference in its entirety.
- Windmiller U.S. Provisional Patent Application No. 62/307,445, filed on Mar. 12, 2016, for a Method And System For Determining Physiological and Environmental Strain Indices is hereby incorporated by reference in its entirety.
- Windmiller U.S. patent application Ser. No. 15/177,289, filed on Jun. 8, 2016, for a Methods And Apparatus For Interfacing A Microneedle-Based Electrochemical Biosensor With An External Wireless Readout Device is hereby incorporated by reference in its entirety.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Engineering & Computer Science (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Heart & Thoracic Surgery (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Medical Informatics (AREA)
- Pathology (AREA)
- Surgery (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Optics & Photonics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Vascular Medicine (AREA)
- Diabetes (AREA)
- Cardiology (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Abstract
A method and device to impart the ability to selectively quantify chemical/biochemical analytes occupying physiological fluids via an automated process that allow the precise and spatially-defined simultaneous deposition of a thin-film of polymer containing an immobilized biorecognition element dispersed therein. A tissue penetrating electrochemical sensor comprises at least one working electrode and at least one of a reference electrode and a counter electrode.
Description
- The Present Application is a continuation application of U.S. patent application Ser. No. 16/152,372, filed on Oct. 4, 2018, which is a continuation application of U.S. patent application Ser. No. 15/590,105, filed on May 9, 2017, now U.S. Pat. No. 10,092,207, issued on Oct. 9, 2018, which claims priority to U.S. Provisional Patent Application No. 62/336,724, filed on May 15, 2016, now expired, each of which is hereby incorporated by reference in its entirety.
- Not Applicable
- The present invention generally relates to electrochemical sensors.
- Electrochemical sensor devices have witnessed increased development activity in recent years, driven primarily by the challenge of in vivo glucose sensing. However, to date, these devices are manufactured using labor- and time-consuming physical assembly processes, especially to impart their selective chemical sensing capabilities. In this vein, such devices have not leveraged high-volume automated manufacturing processes to fabricate electrochemical sensors with high yield and uniformity.
- The prior art discusses various sensors.
- Meyer et al., U.S. Pat. No. 4,756,807 for a Chemically modified electrodes for the catalytic reduction of CO2 discloses an electrode that has a film adsorbed to its surface by reductive electropolymerization of (vbpy)Re(CO)3 Cl (4-vinyl-4′-methyl-2,2′-bipyridine tricarbonylchlororhenium(I)) or its acetonitrile analogue, [(vbpy)Re(CO)3 (MeCN)]+(4-vinyl-4′-methyl-2,2′-bipyridine tricarbonylacetonitrile rhenium (I)). The poly(vbpy)Re(CO)3 Cl or poly[(vbpy)Re(CO)3 (MeCN)]+adsorbed onto the electrode, acts as a catalyst for CO2 reduction to CO. Stability and the reactivity of the polymer film may be increased by co-reductive electropolymerization of either of the aforesaid Re complexes with [(bpy)2 Ru(vpy)2 2+(bis(4-vinyl-pyridine)bis(2,2′-bipyridine) ruthenium (II)) (vpy is 4-vinylpyridine). A bilayer film assembly consisting of poly-[(bpy)2 Ru(vpy)2 ]2+as an inner film and the aforementioned copolymer of Ru/Re yields even greater reactivity to CO2; a method of forming a polymeric film on an electrode; and a method of reducing CO2 to CO using an electrode having a polymeric film adsorbed to its surface.
- Yacynych, U.S. Pat. No. 5,540,828 for a Method for making electrochemical sensors and biosensors having a polymer modified surface discloses a method for making a sensing element for use in a sensor or biosensor that amperometrically measures the concentration of an analyte in a liquid, includes the following sequential steps: a) obtaining an electrode; b) immersing the electrode in a solution of monomer that is capable of being electropolymerized into an electrically insulating polymer; c) flowing an electric current from a cathode through the solution to the electrode at a voltage and amperage sufficient to cause the monomer to polymerize on the surface of the electrode, thereby yielding an electrode coated with an adherent layer of electrically insulating polymer; and e) impregnating the polymeric coating on the surface with a sensing agent that is capable, when contacted by a specific analyte in a chemical or biological liquid, of generating an electroactive molecule that can be detected amperometrically.
- Yacynych et al, U.S. Pat. No. 5,286,364 for a Surface-modified electrochemical biosensor discloses a biosensor.
- Wilson et al., U.S. Pat. No. 6,814,845 for a Method for depositing an enzyme on an electrically conductive substrate discloses improved biosensors are provided having excellent selectivity and stability properties, together with methods of preparing the biosensors. A preferred biosensor includes an electrode having enzyme deposited thereon together with a layer of electropolymerized polymer intermingled with the enzyme; a crosslinked silane film is applied over the polymer layer, and a final coating of polyurethane is formed over the film. In preparative procedures, the enzyme is electrodeposited using an aqueous enzyme solution containing a nonionic surfactant at a concentration level preferably in excess of the critical micelle concentration of the surfactant. In the case of a glucose sensor, the polymer layer is preferably polyphenol, while the silane film is crosslinked (3-aminopropyl) trimethoxysilane. The preferred biosensors have greatly enhanced selectivity stabilities.
- Haesik et al., U.S. Pat. No. 6,413,396 for an Enzyme electrode sensor and manufacturing method thereof discloses an enzyme electrode sensor and a fabricating method thereof, and more particularly, an enzyme electrode sensor which is a biosensor using electrochemical measurement and a manufacturing method thereof. The sensor includes an electrode, a first nonconducting polymer layer formed by electropolymerization outside the electrode wherein enzyme is immobilized in the nonconducting polymer layer, a second nonconducting polymer layer in which enzyme is not immobilized, the second nonconducting layer formed by electropolymerization outside the first nonconducting polymer layer, and an outer layer formed outside the second nonconducting layer. The sensor selectivity is improved as the interference of organic materials is inhibited, and the interference of acetaminophen causing the major problem with a glucose sensor is controlled effectively by the sensor.
- Tissue-penetrating electrochemical sensors represent a promising avenue towards the minimally-invasive quantification of a number of relevant analytes in the physiological fluid, such as interstitial fluid, blood, serum, and plasma. Currently, to impart the ability to selectively quantify chemical/biochemical analytes of interest, membranes comprising of one or more polymers with a biorecognition element dispersed therein are cast in a manual fashion, keeping costs high and sensor-to-sensor uniformity low. In this application, tissue can refer to, but is not limited to, skin, organs, and cells.
- Prior art solutions have been concerned with the immobilization of biorecognition elements by means of entrapment in a polymeric membrane cast by a manual, human-operated process (via dip or drop casting in an aqueous- or solvent-based solution of polymer and enzyme) or by means of the assistance of an automated liquid reagent dispensing robot. This is a serialized process and, as a consequence, low throughput and high device cost are challenges that still confront skin- and tissue-penetrating electrochemical sensor production. When performed by a human operator, such deposition processes often result in a sensor response characterized by a wide degree of variance and hence sensors for clinical applications must often undergo quality control or calibration measures on an individual basis, thereby further impeding the transition to mass production techniques.
- The technology disclosed herein addresses the above challenges by disclosing a method for the automated and parallelized co-deposition of a conducting polymer and biorecognition element at one or more working electrodes of a tissue-penetrating electrochemical sensor.
- The present invention provides for a method to impart the ability to selectively quantify chemical/biochemical analytes occupying physiological fluids via an automated process that allow the precise and spatially-defined simultaneous deposition of a thin-film of polymer containing an immobilized biorecognition element dispersed therein.
- The technology described herein also involves a method for automated, volume-scale functionalization of a tissue-penetrating electrochemical sensor to yield selective chemical or biochemical recognition capability in vivo.
- The present invention delineates a method for the simultaneous co-deposition of both an entrapment polymer and an immobilized biorecognition element onto at least one working electrode using an electro-deposition process for skin- and tissue-penetrating electrochemical sensor functionalization that is highly amenable to high-throughput, automated, parallelized, and highly reproducible manufacturing methods that do away with the need for a human operator during the sensor functionalization process. Indeed, with the trend towards increasing sensor miniaturization, conventional manual processes associated with sensor functionalization to impart a selective biorecognition capability is becoming increasingly inaccessible using a human operator and the need remains for a sensor functionalization strategy that can be exploited along conventional precision manufacturing lines, such as those used for the medical device, MEMS, and semiconductor industries.
- One aspect of the present invention is a tissue-penetrating electrochemical sensor device for the quantification of a chemical or biochemical entity in a physiological fluid. The device comprises a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode, and a conducting polymer film comprising an organic electroactive monomer precursor and at least one biorecognition element. The organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the conducting polymer film. The conducting polymer film is produced by immersing the tissue-penetrating electrochemical sensor in a solution containing the at least one biomolecular recognition element and the organic electroactive monomer precursor which is dissolved in the solution, and wherein application of an oxidizing potential or a reducing potential at the spatially-defined working electrode causes the co-electrodeposition of the conducting polymer film.
- Another aspect of the present invention is a method for the fabrication of a tissue-penetrating electrochemical sensor for the quantification of a chemical or biochemical entity in a physiological fluid. The method includes immersing a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode in a solution comprising at least one biomolecular recognition element and an organic electroactive monomer precursor dissolved in the solution. The method also includes applying of an oxidizing potential or a reducing potential at the spatially-defined working electrode, causing a co-electrodeposition of a polymer film from the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the polymer film.
- The organic electroactive monomer precursor is preferably selected for at least one of its perm-selective properties following conversion to a thin-film of polymer, its charge rejection properties following conversion to a thin-film of polymer, its anti-biofouling properties following conversion to a thin-film of polymer, its porosity following conversion to a thin-film of polymer, its diffusion-limiting nature following conversion to a thin-film of polymer, or its self-limited growth during conversion to a thin-film of polymer.
- The biomolecular recognition element comprises at least one of an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- The tissue-penetrating electrochemical sensor further comprises at least a reference electrode or a counter electrode.
- The oxidizing potential or reducing potential is either a fixed potential or a time-varying potential.
- The oxidizing potential or reducing potential is applied using one of an amperometric technique, a voltammetric technique, a conductometric technique, or a coulometric technique.
- The oxidizing potential or the reducing potential is selected to result in the formation of a precise thickness of the conducting polymer film.
- The oxidizing potential or the reducing potential is applied for a specified time duration to result in the formation of a precise thickness of the conducting polymer film.
- The oxidizing potential or the reducing potential is selected to pass a specified amount of charge through at least one of the working electrode to result in the formation of a precise thickness of the conducting polymer film.
- Having briefly described the present invention, the above and further objects, features and advantages thereof will be recognized by those skilled in the pertinent art from the following detailed description of the invention when taken in conjunction with the accompanying drawings.
-
FIG. 1 is an optical micrograph of an unfunctionalized working electrode. -
FIG. 2 is an optical micrograph of the working electrode shown inFIG. 1 following functionalization. -
FIG. 3 is an electron micrograph delineating the surface morphology of the functionalized working electrode provided inFIG. 2 . -
FIG. 4 is a block/process flow diagram illustrating the major constituents involved in the functionalization of a tissue- or skin-penetrating electrochemical sensor to facilitate chemical or biochemical quantification of various analytes in physiological fluids. -
FIG. 5 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating three-electrode electrochemical sensor. -
FIG. 6 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor. -
FIG. 7 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a multi-component tissue-penetrating three-electrode electrochemical sensor, whereby each electrode is located within an isolated tissue-penetration electrochemical sensor. -
FIG. 8 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor. -
FIG. 9 is a schematic representation delineating the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating electrochemical sensor. - One embodiment is a tissue-penetrating electrochemical sensor device for the quantification of a chemical or biochemical entity in a physiological fluid. The device preferably comprises a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode, and a conducting polymer film comprising an organic electroactive monomer precursor and at least one biorecognition element. The organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the conducting polymer film. The conducting polymer film is produced by immersing the tissue-penetrating electrochemical sensor in a solution containing the at least one biomolecular recognition element and the organic electroactive monomer precursor which is dissolved in the solution, and wherein application of an oxidizing potential or a reducing potential at the spatially-defined working electrode causes the co-electrodeposition of the conducting polymer film.
- Another embodiment is a method for the fabrication of a tissue-penetrating electrochemical sensor for the quantification of a chemical or biochemical entity in a physiological fluid. The method includes immersing a tissue-penetrating electrochemical sensor comprising at least one spatially-defined working electrode in a solution comprising at least one biomolecular recognition element and an organic electroactive monomer precursor dissolved in the solution. The method also includes applying of an oxidizing potential or a reducing potential at the spatially-defined working electrode, causing a co-electrodeposition of a polymer film from the organic electroactive monomer precursor and the at least one biorecognition element dispersed uniformly and physically entrapped in the polymer film.
- The method provides for reliably dispensing a reagent containing at least one biorecognition at the micron-scale, as required to functionalize microelectrodes and microelectrode arrays. The method provides the formation of a polymeric film containing a biorecognition element by means of electro-polymerization enables the precise control of the thickness of the layer as well as the formation of a very thin layer. Past conventional methods of forming a polymeric layer by use of drop casting, dip coating, spin coating, or dispensing had the difficulty of controlling the thickness of the layer, thereby forming a relatively thick and non-uniform layer. Therefore, electro-polymerization utilized in the present invention is very effective for the reproducible formation of a layer as well as the simultaneous realization of co-deposition of both the polymer and the biorecognition element.
- In addition to variations in film thickness caused by conventional sensor functionalization approaches, these techniques require multiple steps to create a selective, diffusion-limiting, interference-rejecting sensor membrane and it is the case that a polymeric film is cast on the electrode following a prior biorecognition element deposition step, which further contributes to decreased sensor uniformity and increased manufacturing cost and time. The simultaneous co-deposition of both the polymer and the biorecognition element, as disclosed in the current invention, serve to mitigate these challenges that have plagued the electrochemical sensor industry for some time.
- Precisely-defined thin-films, whose thickness and porosity can be precisely controlled by the suitable application of voltage, current, or charge that is passed through the electrode, also embody the advantage that the approach requires minimal quantity of reagent, thereby contributing to a reduction in cost to manufacture.
- The technology disclosed herein discloses a method for the automated and parallelized co-deposition of a conducting polymer and biorecognition element at one or more working electrodes of a tissue-penetrating electrochemical sensor. The method involves the immersion of the electrochemical sensor in a solution of an organic electroactive monomeric precursor (to the conducting polymer) and biorecognition element (enzyme, antibody, etc.) and applying a fixed- or time-varying potential at one or more of the working electrodes to instigate co-electrodeposition. This encourages the electro-polymerization of a conducting polymer film, synthesized from the monomeric precursor, onto the working electrode surface. Because it is uniformly dispersed in solution and often is a large molecule (larger than the pore size of the polymer), the biorecognition element is also entrapped, in a spatially uniform fashion, in the polymer film and can, optionally, serve as the counter-ion or dopant during the electro-polymerization process through suitable choice of a biorecognition element with appropriate isoelectric point and solution pH.
- Leveraging this self-assembly process, which is also self-limiting, the thickness and porosity of the polymeric film is adjusted via modification of the applied potential, current, or charge passed through the electrochemical cell during the co-electrodeposition process. This has wide-ranging implications pertaining to the sensitivity, selectivity, stability, and response time of the as-synthesized tissue-penetrating electrochemical sensor.
- The disclosed technique facilitates spatially-defined deposition of precisely-controlled thin-films on one or more working electrode surfaces and allows the sensor designer to synthesize entrapment membranes to achieve certain utilitarian features such as co-circulating electroactive interference rejection (via perm- or charge-selectivity), increased linearity, extended lifetime, minimal signal drift, or reduced tendency to undergo biofouling when used in vivo.
- Simple and reproducible formations of chemically- and biochemically-selective polymer films can be formed with the disclosed method, which obviates the need for multiple biorecognition element casting and polymer casting steps by allowing for the simultaneous co-deposition of a thin-film containing both an entrapping polymer and biorecognition element. As such, the as-disclosed sensor functionalization strategy enables parallelized sensor functionalization without human intervention and is amenable to implementation along assembly lines or semiconductor fabrication processes.
- The following commercially-available components are used in the synthesis of the sensor:
- The voltage/current. source is preferably a CH INSTRUMENTS Model 10000 Series Multi-Potentiostat, Metrohm Autolab PGSTAT Series Potentiostat Galvanostat, or Palmsens EmStat Potentiostat. The voltages utilized depend on the film, and generally range from −0.2 volts to +1.0 volts. The current transduced ranges from 10 picoAmperes (pA) to 100 microAmperes (μA), and is dependent on whether a singular electrode is electro-deposited or if multiple electrodes are electro-deposited as a group.
- The working electrode is preferably a
CH INSTRUMENTS CHI101 2 mm-diameter gold disc microelectrode, or a METROHM MF-2150 100 μm-diameter platinum disc microelectrode. - The tissue-penetrating electrochemical sensor is preferably a subcutaneous, percutaneous, transdermal, or intradermal sensor in which an electrical stimulus is applied to encourage a redox reaction and in which a voltage, current, charge, resistance, or impedance property is measured to infer the concentration of a particular chemical/biochemical analyte of interest present in the physiological fluid compartment in which the sensor is located. The sensor also comprises at least one working electrode (defined below) and at least one of a counter electrode and a reference electrode.
- The working electrode is preferably a spatially-defined electrode whereby the electrochemical sensing operation is to occur; functionalized with a polymer and biorecognition element to impart the selective ability to transduce the chemical/biochemical of interest into an electrical signal. A defined voltage, current, or amount of charge is applied as a stimulus to the working electrode to instigate a redox reaction and a voltage, current, charge, resistance, or impedance response is measured to infer the concentration of a particular chemical/biochemical analyte of interest.
- The organic electroactive monomer precursor is preferably an organic monomer that is either oxidized or reduced by the application of a suitable voltage, current, or amount of charge on a working electrode surface and concomitantly electro-deposited/electro-polymerized on the working electrode surface from a solution in which it is dissolved.
- The biorecognition element is preferably an organic, inorganic, or biological compound that selectively binds, catalyzes, or sieves a particular chemical/biochemical analyte of interest and, in doing so, changes its electrical properties (voltage, current, charge, resistance, or impedance) or produces an electroactive product (H2O2, NADH, etc.). Examples of a biorecognition element include, but are not limited to, an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- The conducting polymer is preferably synthesized from the organic electroactive monomer precursor through the process of electro-polymerization. The conducting polymer serves to physically entrap the biorecognition element in its highly porous matrix, thereby forming a thin-film with selective sensing capabilities. Alternatively, the biorecognition element can serve as the dopant or counter-ion through the appropriate selection of the biorecognition element's isoelectric point and solution pH in which the organic electroactive monomer precursor and biorecognition element are dissolved. The polymer can also be selected to exhibit perm-selective, charge-selective, size-selective, or anti-biofouling properties. The polymer film thickness preferably ranges from 5 nanometers (“nm”) to 50 nm. The thickness is primarily governed by the voltage applied, the time that the voltage is applied, the amount of the monomeric precursor in the solution, and the geometry of the electrode. The porosity of the film and the perm-selectivity of the film are also adjusted by these factors.
- The voltage or current source is either a power supply, potentiostat, galvanostat, function generator, or any source of electrical voltage or current. The voltage or current source is operated to apply a particular voltage, current, or amount of charge. The voltage or current source is terminated once a certain amount of time has transpired or charge has passed through the working electrode. The voltages utilized depend on the film and generally range from −0.2 volts to +1.0 volts. The current transduced ranges from 10 picoAmperes (pA) to 100 microAmperes (μA), and is dependent on whether a singular electrode is electro-deposited or if multiple electrodes are electro-deposited as a group.
- The solution composition comprises the organic electroactive monomeric precursor dissolved in H20 and optionally salt(s) from the group consisting of NaCl, KCl, MgSO4, CaCl2, Na2HPO4, NaH2PO4, K2HPO4, and KH2PO4. The organic monomeric precursor is preferably one or more of, but not limited to, the following: aniline, pyrrole, acetylene, phenylene, phenylene vinylene, phenylene diamine, thiophene, 3,4-ethylenedioxythiophene, aminophenylboronic acid.
- The method steps of the invention include the following: 1) Immersion of a tissue-penetrating electrochemical sensor in a solution of at least one of a biorecognition element and an organic electroactive monomer precursor. A tissue-penetrating
electrochemical sensor 100 containing, in this example, exactly onecounter electrode 101, one workingelectrode 102, and onereference electrode 103 is immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. In a two-electrode embodiment, a tissue-penetratingelectrochemical sensor 100 containing, in this example, exactly one workingelectrode 102 and onereference electrode 103 is immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. 2) Application of an oxidizing or reducing potential upon at least one working electrode contained in electrochemical sensor. A voltage orcurrent source 105 is connected, in an ohmic fashion, to counterelectrode 101 and at least one of 102 and 103; a constant or time-varying potential is applied to counterelectrode 101 to pass a controlled and pre-determined amount of current or charge throughcounter electrode 101. 3) Simultaneous co-electrodeposition of a thin-film of polymer comprised of organic electroactive monomer precursor and the at least one biorecognition element. Following the application of a potential, current, or charge, the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, serves to entrap the biomolecular recognition element in the highly porous matrix created therein. This processes functionalizes the workingelectrode 102 of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. - The inputs of the invention are as follow:
- 1) an organic electroactive monomer precursor. The organic monomer is either oxidized or reduced by the application of a suitable voltage, current, or amount of charge on a working electrode surface and concomitantly electro-deposited/electro-polymerized on the working electrode surface from a solution in which it is dissolved.
- 2) Biorecognition element. An organic, inorganic, or biological compound that selectively binds, catalyzes, or sieves a particular chemical/biochemical analyte of interest and, in doing so, changes its electrical properties (voltage, current, charge, resistance, or impedance) or produces an electroactive product (H2O2, NADH, etc.). Examples of a biorecognition element include, but are not limited to, an enzyme, biocatalyst, inorganic catalyst, ion-selective material, antibody, oligonucleotide, electrochemical redox mediator, cell, or organelle.
- 3) Electrical stimulus. A specified and controlled voltage, current, or amount of charge is applied. The electrical stimulus is terminated once a certain amount of time has transpired or charge has passed through the system.
- The outputs of the invention are as follows: Functionalized working electrode. One or more working electrodes are coated with a thin-film, typically on the order of 1-100 nm, of the polymerized organic
electroactive monomer 110, which serves to entrap the biomolecular recognition element to form a co-deposited, highly porous polymer-biorecognition element matrix 109. This processes functionalizes the workingelectrode 102 of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. -
FIG. 1 is an optical micrograph of an unfunctionalized working electrode. -
FIG. 2 is an optical micrograph of the working electrode shown inFIG. 1 following functionalization. The presence of a thin-film of co-electrodeposited polymer and enzyme can be observed from the image. -
FIG. 3 is an electron micrograph delineating the surface morphology of the functionalized working electrode provided inFIG. 2 . -
FIG. 4 is a block/process flow diagram illustrating the major constituents involved in the functionalization of a tissue- or skin-penetrating electrochemical sensor to facilitate chemical or biochemical quantification of various analytes in physiological fluids. -
FIG. 5 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating three-electrode electrochemical sensor. Briefly, a tissue-penetratingelectrochemical sensor 100 containing, in this example, exactly onecounter electrode 101, one workingelectrode 102, and onereference electrode 103 is immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. A voltage orcurrent source 105 is connected, in an ohmic fashion, to 102 and at least one of 101 and 103; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102. This, in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein. This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. -
FIG. 6 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor. Briefly, a tissue-penetratingelectrochemical sensor 100 containing, in this example, exactly one workingelectrode 102 and onereference electrode 103 is immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. A voltage orcurrent source 105 is connected, in an ohmic fashion, to 102 and 103; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102. This, in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein. This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. -
FIG. 7 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a multi-component tissue-penetrating three-electrode electrochemical sensor, whereby each electrode is located within an isolated tissue-penetration electrochemical sensor. Briefly, three tissue-penetratingelectrochemical sensors 100 containing, in this example, exactly onecounter electrode 101, one workingelectrode 102, and onereference electrode 103 are immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. A voltage orcurrent source 105 is connected, in an ohmic fashion, to 102 and at least one of 101 and 103; a constant or time-varying potential is applied to 102 to pass a controlled and pre-determined amount of current or charge through 102. This, in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein. This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. -
FIG. 8 is a schematic illustration delineating the major steps involved in the co-electrodeposition of a polymer-biorecognition element film on at least one working electrode of a tissue-penetrating two-electrode electrochemical sensor. Briefly, two tissue-penetratingelectrochemical sensors 100 containing, in this example, exactly one workingelectrode 102 and onereference electrode 103 are immersed in asolution 104 comprising at least one of a biomolecular recognition element and at least one of an organic electroactive monomer precursor dissolved in said solution. A voltage orcurrent source 105 is connected, in an ohmic fashion, to workingelectrode 102 andreference electrode 103; a constant or time-varying potential is applied to workingelectrode 102 to pass a controlled and pre-determined amount of current or charge through workingelectrode 102. This, in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer, which serves to entrap the said biomolecular recognition element in the highly porous matrix created therein. This processes functionalizes the working electrode of the tissue-penetrating electrochemical sensor and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. -
FIG. 9 is a schematic representation delineating the co-electrodeposition of a polymer-biorecognition element film on at least one workingelectrode 102 of a tissue-penetrating electrochemical sensor. The workingelectrode 102 in this example is immersed in asolution 104 comprising at least one of a biomolecular recognition element (107 in inset) and at least one of an organic electroactive monomer precursor (108 in inset) dissolved in said solution. A voltage orcurrent source 105 is connected, in an ohmic fashion, to workingelectrode 102; a constant or time-varying potential is applied to workingelectrode 102 to pass a controlled and pre-determined amount of current or charge through workingelectrode 102. This, in turn, instigates the formation of a thin-film, typically on the order of 1-100 nm, of the polymerized organic electroactive monomer (110 in inset), which serves to entrap the biomolecular recognition element to form a co-deposited, highly porous polymer-biorecognition element matrix 109. This processes functionalizes the workingelectrode 102 of the tissue-penetratingelectrochemical sensor 100 and allows it to achieve the selective quantification of at least one chemical or biochemical analyte in a physiological fluid. - McCanna et al., U.S. patent application Ser. No. 14/843,926, filed on Sep. 2, 2015, for a Miniaturized Sub-Nanoampere Sensitivity Low-Noise Potentiostat System is hereby incorporated by reference in its entirety.
- Windmiller, U.S. Provisional Patent Application No. 62/233,323, filed on Sep. 26, 2015, for a Methods And Devices For Embedding Sensors In Container Labels and Packaging is hereby incorporated by reference in its entirety.
- Windmiller et al., U.S. Provisional Patent Application No. 62/233,324, filed on Sep. 26, 2015, for a Method And System For Manufacturing Microneedles With Electrodes is hereby incorporated by reference in its entirety.
- Windmiller et al., U.S. Provisional Patent Application No. 62/247,732, filed on Oct. 28, 2015, for a Method And System For Three Dimensional Printing Of Microneedles And Microneedle Arrays For Transdermal Biosensing And Drug Delivery is hereby incorporated by reference in its entirety.
- Windmiller et al., U.S. Provisional Patent Application No. 62/247,730, filed on Oct. 28, 2015, for a Sensors For Wearable Devices is hereby incorporated by reference in its entirety.
- Windmiller et al., U.S. patent application Ser. No. 14/955,850, filed on Dec. 1, 2015, for a Method And Apparatus For Determining Body Fluid Loss is hereby incorporated by reference in its entirety.
- Windmiller, U.S. Provisional Patent Application No. 62/307,444, filed on Mar. 12, 2016, for a Method And Apparatus For Determining Exhaled Carbon Loss is hereby incorporated by reference in its entirety.
- Windmiller, U.S. Provisional Patent Application No. 62/307,445, filed on Mar. 12, 2016, for a Method And System For Determining Physiological and Environmental Strain Indices is hereby incorporated by reference in its entirety.
- Windmiller, U.S. patent application Ser. No. 15/177,289, filed on Jun. 8, 2016, for a Methods And Apparatus For Interfacing A Microneedle-Based Electrochemical Biosensor With An External Wireless Readout Device is hereby incorporated by reference in its entirety.
- Wang et al., U.S. Patent Publication Number 20140336487 for a Microneedle Arrays For Biosensing And Drug Delivery is hereby incorporated by reference in its entirety.
- From the foregoing it is believed that those skilled in the pertinent art will recognize the meritorious advancement of this invention and will readily understand that while the present invention has been described in association with a preferred embodiment thereof, and other embodiments illustrated in the accompanying drawings, numerous changes modification and substitutions of equivalents may be made therein without departing from the spirit and scope of this invention which is intended to be unlimited by the foregoing except as may appear in the following appended claim. Therefore, the embodiments of the invention in which an exclusive property or privilege is claimed are defined in the following appended claims.
Claims (21)
1-20. (canceled)
21. A method of fabricating a tissue-penetrating electrochemical sensor for quantification of a chemical entity or a biochemical entity in a physiological fluid, the method comprising:
applying a conducting polymer film to a surface of a working electrode, the application comprising:
immersing an array of microelectrodes in a solution comprising a biorecognition element and a monomer precursor, the array of microelectrodes comprising the working electrode; and
applying, during the immersion of the array of microelectrodes in the solution, a potential at the working electrode, the application of the potential resulting in an electrodeposition of the conducting polymer film on the surface of the working electrode.
22. The method of claim 21 , wherein the conducting polymer film is synthesized from the monomer precursor.
23. The method of claim 22 , wherein the biorecognition element is entrapped and dispersed uniformly in the conducting polymer film.
24. The method of claim 21 , wherein the monomer precursor is dissolved in the solution.
25. The method of claim 21 , wherein the electrodeposition of the conducting polymer film comprises a co-electrodeposition of the biorecognition element and the monomer precursor.
26. The method of claim 21 , wherein the biorecognition element comprises at least one of an enzyme, a biocatalyst, an inorganic catalyst, an ion-selective material, an antibody, aptamer, an oligonucleotide, an electrochemical redox mediator, a cell, and an organelle.
27. The method of claim 21 , wherein the monomer precursor comprises an organic monomer.
28. The method of claim 21 , wherein the potential comprises a fixed potential or a time-varying potential.
29. The method of claim 28 , wherein the potential is selected to result in a thickness of the conducting polymer film ranging from about 5 to about 5,000 nanometers.
30. The method of claim 21 , wherein the potential is applied for a specified time duration to result in a thickness of the conducting polymer film ranging from about 5 to about 5,000 nanometers.
31. The method of claim 21 , wherein the potential is selected to pass a specified amount of charge through the working electrode to result in a thickness of the conducting polymer film ranging from about 5 to about 5,000 nanometers.
32. The method of claim 21 , wherein the array of microelectrodes further comprises another electrode, wherein the application of the potential comprises applying the potential between the working electrode and the other electrode.
33. The method of claim 32 , wherein the other electrode comprises a counter electrode or a reference electrode.
34. The method of claim 21 , wherein the array of microelectrodes further comprises a counter electrode and a reference electrode, wherein the application of the potential comprises applying the potential between the working electrode and at least one of the counter electrode and the reference electrode.
35. The method of claim 21 , wherein the array of microelectrodes comprises a plurality of working electrodes, wherein the application of the potential comprises applying the potential at each of the plurality of working electrodes, the application of the potential resulting in the electrodeposition of the conducting polymer film on a respective surface of each of the plurality of working electrodes.
36. A method of fabricating a tissue-penetrating electrochemical sensor for quantification of a chemical entity or a biochemical entity in a physiological fluid, the method comprising:
applying a conducting polymer film to a respective surface of each of a plurality of working electrodes, the application comprising:
immersing an array of microelectrodes in a solution comprising a biorecognition element and a monomer precursor, the array of microelectrodes comprising the plurality of working electrodes; and
applying, during the immersion of the array of microelectrodes in the solution, a potential at the plurality of working electrodes, the application of the potential resulting in an electrodeposition of the conducting polymer film on the respective surface of each of the plurality of working electrodes.
37. A tissue-penetrating electrochemical sensor device for quantification of a chemical entity or a biochemical entity in a physiological fluid, the device comprising:
an array of microelectrodes comprising a plurality of working electrodes; and
a conducting polymer film on a respective surface of each of the plurality of working electrodes, the conducting polymer film comprising a monomer precursor and a biorecognition element, the conducting polymer film electrodeposited on the respective surface of each of the plurality of working electrodes in response to immersion of the plurality of working electrodes in a solution and application of a potential applied to each of the plurality of working electrodes, the solution comprising the biorecognition element and the monomer precursor.
38. The device of claim 37 , wherein the conducting polymer film has a thickness of about 1 to about 100 nanometers.
39. The device of claim 37 , wherein the conducting polymer film is synthesized from the monomer precursor, wherein the biorecognition element is entrapped and dispersed uniformly in the conducting polymer film, and wherein the monomer precursor is dissolved in the solution.
40. The device of claim 37 , wherein the potential applied to each of the plurality of working electrodes ranges from −0.5 volts to 1.5 volts.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17/850,832 US20230137258A1 (en) | 2016-05-15 | 2022-06-27 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662336724P | 2016-05-15 | 2016-05-15 | |
| US15/590,105 US10092207B1 (en) | 2016-05-15 | 2017-05-09 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/152,372 US10492708B1 (en) | 2016-05-15 | 2018-10-04 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/666,259 US11406818B2 (en) | 2016-05-15 | 2019-10-28 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US17/850,832 US20230137258A1 (en) | 2016-05-15 | 2022-06-27 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/666,259 Continuation US11406818B2 (en) | 2016-05-15 | 2019-10-28 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20230137258A1 true US20230137258A1 (en) | 2023-05-04 |
Family
ID=63685194
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/590,105 Active 2037-06-28 US10092207B1 (en) | 2016-05-15 | 2017-05-09 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/152,372 Active US10492708B1 (en) | 2016-05-15 | 2018-10-04 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/666,259 Active US11406818B2 (en) | 2016-05-15 | 2019-10-28 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US17/850,832 Pending US20230137258A1 (en) | 2016-05-15 | 2022-06-27 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/590,105 Active 2037-06-28 US10092207B1 (en) | 2016-05-15 | 2017-05-09 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/152,372 Active US10492708B1 (en) | 2016-05-15 | 2018-10-04 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| US16/666,259 Active US11406818B2 (en) | 2016-05-15 | 2019-10-28 | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
Country Status (1)
| Country | Link |
|---|---|
| US (4) | US10092207B1 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| USD996999S1 (en) | 2021-11-16 | 2023-08-29 | Biolinq Incorporated | Wearable sensor |
| US11857344B2 (en) | 2021-05-08 | 2024-01-02 | Biolinq Incorporated | Fault detection for microneedle array based continuous analyte monitoring device |
| US11872055B2 (en) | 2020-07-29 | 2024-01-16 | Biolinq Incorporated | Continuous analyte monitoring system with microneedle array |
| USD1012744S1 (en) | 2022-05-16 | 2024-01-30 | Biolinq Incorporated | Wearable sensor with illuminated display |
| USD1013544S1 (en) | 2022-04-29 | 2024-02-06 | Biolinq Incorporated | Wearable sensor |
| US11904127B2 (en) | 2021-09-28 | 2024-02-20 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11963796B1 (en) | 2017-04-29 | 2024-04-23 | Biolinq Incorporated | Heterogeneous integration of silicon-fabricated solid microneedle sensors and CMOS circuitry |
| USD1033641S1 (en) | 2021-12-17 | 2024-07-02 | Biolinq Incorporated | Microneedle array sensor applicator device |
| USD1035004S1 (en) | 2023-02-28 | 2024-07-09 | Biolinq Incorporated | Wearable sensor |
| US12070313B2 (en) | 2022-07-05 | 2024-08-27 | Biolinq Incorporated | Sensor assembly of a microneedle array-based continuous analyte monitoring device |
| US12109032B1 (en) | 2017-03-11 | 2024-10-08 | Biolinq Incorporated | Methods for achieving an isolated electrical interface between an anterior surface of a microneedle structure and a posterior surface of a support structure |
| USD1051745S1 (en) | 2023-04-27 | 2024-11-19 | Biolinq Incorporated | Wearable sensor |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10092207B1 (en) * | 2016-05-15 | 2018-10-09 | Biolinq, Inc. | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| USD875254S1 (en) * | 2018-06-08 | 2020-02-11 | Biolinq, Inc. | Intradermal biosensor |
| US11576586B2 (en) | 2018-12-18 | 2023-02-14 | Movano Inc. | Methods for radio wave based health monitoring that utilize data derived from amplitude and/or phase data |
| US11366197B2 (en) | 2018-12-18 | 2022-06-21 | Movano Inc. | Methods for operating stepped frequency radar systems with digital demultiplexing |
| US11540774B2 (en) | 2018-12-18 | 2023-01-03 | Movano Inc. | Removable smartphone case for radio wave based health monitoring |
| US11992299B2 (en) | 2018-12-18 | 2024-05-28 | Movano Inc. | Wearable devices for health monitoring using radio waves that include signal isolation |
| EP4164489A4 (en) * | 2020-06-10 | 2024-06-19 | Zense-Life Inc. | Gas sterilized continuous metabolic monitor |
| US20220175278A1 (en) * | 2020-12-03 | 2022-06-09 | Biolinq Incorporated | Solid-state substrate-integrated reference electrode and counter electrode |
| US20240035065A1 (en) * | 2020-12-07 | 2024-02-01 | The University Of North Carolina At Chapel Hill | Method for measurement in biosensors |
| US11864861B2 (en) | 2020-12-18 | 2024-01-09 | Movano Inc. | Method for monitoring a physiological parameter in a person that involves spectral agility |
| US11883134B2 (en) | 2020-12-18 | 2024-01-30 | Movano Inc. | System for monitoring a physiological parameter in a person that involves coherently combining data generated from an RF-based sensor system |
| US11786133B2 (en) | 2020-12-18 | 2023-10-17 | Movano Inc. | System for monitoring a health parameter of a person utilizing a pulse wave signal |
| US11832919B2 (en) | 2020-12-18 | 2023-12-05 | Movano Inc. | Method for generating training data for use in monitoring the blood pressure of a person that utilizes a pulse wave signal generated from radio frequency scanning |
| US12121336B2 (en) | 2020-12-18 | 2024-10-22 | Movano Inc. | Method for monitoring a physiological parameter in a person that involves coherently combining data generated from an RF-based sensor system |
| USD988160S1 (en) | 2021-03-16 | 2023-06-06 | Biolinq Incorporated | Wearable dermal sensor |
| JP2024527580A (en) | 2021-07-07 | 2024-07-25 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | Wearable non-invasive microneedle sensor |
| US20240074682A1 (en) * | 2022-09-02 | 2024-03-07 | Dexcom, Inc. | Devices and methods for measuring a concentration of a target analyte in a biological fluid in vivo |
| US20240315614A1 (en) | 2023-02-02 | 2024-09-26 | Biolinq Incorporated | Method for improved sensor sensitivity of a microneedle-based continuous analyte monitoring system |
Family Cites Families (122)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4305401A (en) | 1979-05-16 | 1981-12-15 | Hughes Aircraft Company | Digital watch/infrared plethysmograph having a quick release remote pulse sensor having a finger cuff |
| DE2940246A1 (en) | 1979-10-04 | 1981-04-16 | Gebrüder Junghans GmbH, 7230 Schramberg | ELECTRONIC WRISTWATCH WITH ELECTRICAL CONNECTION CONTACTS |
| US4450842A (en) | 1980-04-25 | 1984-05-29 | Cordis Corporation | Solid state reference electrode |
| US4407295A (en) | 1980-10-16 | 1983-10-04 | Dna Medical, Inc. | Miniature physiological monitor with interchangeable sensors |
| US4756807A (en) | 1986-10-09 | 1988-07-12 | Gas Research Institute | Chemically modified electrodes for the catalytic reduction of CO2 |
| US4908117A (en) | 1986-11-13 | 1990-03-13 | Monsanto Company | Solid state reference electrode |
| US5286364A (en) | 1987-06-08 | 1994-02-15 | Rutgers University | Surface-modified electochemical biosensor |
| US5540828A (en) | 1987-06-08 | 1996-07-30 | Yacynych; Alexander | Method for making electrochemical sensors and biosensors having a polymer modified surface |
| WO1989006855A1 (en) | 1988-01-21 | 1989-07-27 | Electro-Nucleonics, Inc. | Dry ion-selective electrodes for the determination of ionic species in aqueous media |
| US5362307A (en) | 1989-01-24 | 1994-11-08 | The Regents Of The University Of California | Method for the iontophoretic non-invasive-determination of the in vivo concentration level of an inorganic or organic substance |
| KR970011449B1 (en) | 1988-01-29 | 1997-07-11 | 더 리전트 오브 디 유니버시티 오브 캘리포니아 | Device for iontophoretic non-invasive sampling or delivery of substances |
| US5131390A (en) | 1989-09-14 | 1992-07-21 | Suzuken Co. | Device for continuously measuring the skin local sweating rate |
| US5166063A (en) | 1990-06-29 | 1992-11-24 | Eli Lilly And Company | Immobolization of biomolecules by enhanced electrophoretic precipitation |
| JP3492086B2 (en) | 1995-06-30 | 2004-02-03 | セイコーエプソン株式会社 | Wrist-mounted pulse wave measuring device and pulse wave information processing device |
| US6036055A (en) | 1996-11-12 | 2000-03-14 | Barmate Corporation | Wireless liquid portion and inventory control system |
| US6139718A (en) | 1997-03-25 | 2000-10-31 | Cygnus, Inc. | Electrode with improved signal to noise ratio |
| US7220550B2 (en) | 1997-05-14 | 2007-05-22 | Keensense, Inc. | Molecular wire injection sensors |
| US8974386B2 (en) | 1998-04-30 | 2015-03-10 | Abbott Diabetes Care Inc. | Analyte monitoring device and methods of use |
| US7344499B1 (en) | 1998-06-10 | 2008-03-18 | Georgia Tech Research Corporation | Microneedle device for extraction and sensing of bodily fluids |
| US6132449A (en) | 1999-03-08 | 2000-10-17 | Agilent Technologies, Inc. | Extraction and transportation of blood for analysis |
| JP3611983B2 (en) | 1999-04-06 | 2005-01-19 | セイコーインスツル株式会社 | Small electronic equipment with sensor |
| US6312612B1 (en) | 1999-06-09 | 2001-11-06 | The Procter & Gamble Company | Apparatus and method for manufacturing an intracutaneous microneedle array |
| KR100360774B1 (en) | 1999-12-27 | 2002-11-13 | 한국전자통신연구원 | Enzyme electrode sensor and manufacturing method thereof |
| AU2001275138A1 (en) | 2000-06-02 | 2001-12-17 | The University Of Utah Research Foundation | Active needle devices with integrated functionality |
| US6603987B2 (en) | 2000-07-11 | 2003-08-05 | Bayer Corporation | Hollow microneedle patch |
| CA2424520C (en) | 2000-08-21 | 2007-01-02 | The Cleveland Clinic Foundation | Microneedle array module and method of fabricating the same |
| US20020026111A1 (en) | 2000-08-28 | 2002-02-28 | Neil Ackerman | Methods of monitoring glucose levels in a subject and uses thereof |
| US6793789B2 (en) | 2000-09-30 | 2004-09-21 | Geun Sig Cha | Reference electrode with a polymeric reference electrode membrane |
| US20030116447A1 (en) | 2001-11-16 | 2003-06-26 | Surridge Nigel A. | Electrodes, methods, apparatuses comprising micro-electrode arrays |
| US6814845B2 (en) | 2001-11-21 | 2004-11-09 | University Of Kansas | Method for depositing an enzyme on an electrically conductive substrate |
| US6908453B2 (en) | 2002-01-15 | 2005-06-21 | 3M Innovative Properties Company | Microneedle devices and methods of manufacture |
| CA2929300C (en) | 2002-03-11 | 2019-04-16 | Nitto Denko Corporation | Transdermal drug delivery patch system, method of making same and method of using same |
| US7343188B2 (en) | 2002-05-09 | 2008-03-11 | Lifescan, Inc. | Devices and methods for accessing and analyzing physiological fluid |
| US7415299B2 (en) | 2003-04-18 | 2008-08-19 | The Regents Of The University Of California | Monitoring method and/or apparatus |
| US7169284B1 (en) | 2003-09-22 | 2007-01-30 | Pacesetter, Inc. | High surface area cathode for electrolytic capacitors using conductive polymer |
| US20050109622A1 (en) | 2003-11-26 | 2005-05-26 | Peter Peumans | Method for controlling electrodeposition of an entity and devices incorporating the immobilized entity |
| GB2411719B (en) | 2004-03-04 | 2008-02-06 | Leon Thomas Lee Marsh | Hydration monitor |
| TWI246929B (en) | 2004-07-16 | 2006-01-11 | Ind Tech Res Inst | Microneedle array device and its fabrication method |
| US7132054B1 (en) | 2004-09-08 | 2006-11-07 | Sandia Corporation | Method to fabricate hollow microneedle arrays |
| US7097776B2 (en) | 2004-10-22 | 2006-08-29 | Hewlett-Packard Development Company, L.P. | Method of fabricating microneedles |
| GB2423075B (en) | 2004-12-01 | 2009-07-08 | Univ The Arts London | A system and method for dispensing fluid in response to a sensed property |
| EP1824655B1 (en) | 2004-12-07 | 2010-05-26 | 3M Innovative Properties Company | Method of molding a microneedle |
| KR20060089314A (en) | 2005-02-03 | 2006-08-09 | 삼성전자주식회사 | Method for fabrication of micor needle array |
| PL206043B1 (en) | 2005-03-01 | 2010-06-30 | Teresa Błaż | Reference electrode for the electro-gravimetric measurements, particularly for potentiometric measurements |
| CA2602259A1 (en) | 2005-03-29 | 2006-10-05 | Arkal Medical, Inc. | Devices, systems, methods and tools for continuous glucose monitoring |
| US8744546B2 (en) | 2005-05-05 | 2014-06-03 | Dexcom, Inc. | Cellulosic-based resistance domain for an analyte sensor |
| ATE555824T1 (en) | 2005-04-11 | 2012-05-15 | Infotonics Technology Ct Inc | BLOOD MONITORING SYSTEMS AND METHODS THEREOF |
| US8060174B2 (en) | 2005-04-15 | 2011-11-15 | Dexcom, Inc. | Analyte sensing biointerface |
| EP1874179A2 (en) | 2005-04-25 | 2008-01-09 | Infotonics Technology Center, Inc. | Microneedle with glucose sensor and method thereof |
| US20090143761A1 (en) | 2005-06-03 | 2009-06-04 | Transdermal Patents Company, Llc | Agent delivery system and uses of same |
| US9084546B2 (en) * | 2005-08-31 | 2015-07-21 | The Regents Of The University Of Michigan | Co-electrodeposited hydrogel-conducting polymer electrodes for biomedical applications |
| US8005526B2 (en) | 2005-08-31 | 2011-08-23 | The Regents Of The University Of Michigan | Biologically integrated electrode devices |
| US7837654B2 (en) | 2005-12-15 | 2010-11-23 | The United States Of America As Represented By The Secretary Of The Army | Precision sensing and treatment delivery device for promoting healing in living tissue |
| EP1820441A1 (en) | 2006-02-16 | 2007-08-22 | Roche Diagnostics GmbH | Microneedle arrays with attenuated total reflection (ATR) sensor |
| US20070213044A1 (en) | 2006-03-01 | 2007-09-13 | The Regents Of The University Of California | Wireless nanoamp resolution galvanostat/potentialstat |
| US20080154107A1 (en) | 2006-12-20 | 2008-06-26 | Jina Arvind N | Device, systems, methods and tools for continuous glucose monitoring |
| US20100049021A1 (en) | 2006-03-28 | 2010-02-25 | Jina Arvind N | Devices, systems, methods and tools for continuous analyte monitoring |
| US20090131778A1 (en) | 2006-03-28 | 2009-05-21 | Jina Arvind N | Devices, systems, methods and tools for continuous glucose monitoring |
| US20070282246A1 (en) | 2006-06-05 | 2007-12-06 | Mit, Llp | Iontosonic-microneedle biosensor apparatus and methods |
| US20080097352A1 (en) | 2006-09-12 | 2008-04-24 | Beck Patricia A | Methods of fabricating microneedles with bio-sensory functionality |
| US8022292B2 (en) | 2006-10-02 | 2011-09-20 | SolASE Corporation | Photovoltaic device employing a resonator cavity |
| TW200829215A (en) | 2007-01-03 | 2008-07-16 | Univ Nat Chiao Tung | Micro probe array and manufacturing method of the trans-print mold thereof |
| US20080234562A1 (en) | 2007-03-19 | 2008-09-25 | Jina Arvind N | Continuous analyte monitor with multi-point self-calibration |
| US7493232B1 (en) | 2007-08-28 | 2009-02-17 | Surina Blake J | Device and method for monitoring hydration status |
| GB2452714A (en) | 2007-09-11 | 2009-03-18 | Eleksen Ltd | Intelligent connector for interfacing fabric sensors with processing devices |
| US20090088652A1 (en) | 2007-09-28 | 2009-04-02 | Kathleen Tremblay | Physiological sensor placement and signal transmission device |
| MY157968A (en) | 2007-11-14 | 2016-08-30 | Mimos Berhad | Method for fabricating microneedled and microneedle fabricated from the same |
| WO2009124095A1 (en) | 2008-03-31 | 2009-10-08 | Abbott Diabetes Care Inc. | Shallow implantable analyte sensor with rapid physiological response |
| US7893701B2 (en) | 2008-05-05 | 2011-02-22 | Formfactor, Inc. | Method and apparatus for enhanced probe card architecture |
| US9387000B2 (en) | 2008-05-23 | 2016-07-12 | The University Of Queensland | Analyte detection using a needle projection patch |
| GB0811874D0 (en) | 2008-06-30 | 2008-07-30 | Nemaura Pharma Ltd | Patches for reverse iontophoresis |
| US20100056873A1 (en) | 2008-08-27 | 2010-03-04 | Allen Paul G | Health-related signaling via wearable items |
| US8284046B2 (en) | 2008-08-27 | 2012-10-09 | The Invention Science Fund I, Llc | Health-related signaling via wearable items |
| US8094009B2 (en) | 2008-08-27 | 2012-01-10 | The Invention Science Fund I, Llc | Health-related signaling via wearable items |
| US8130095B2 (en) | 2008-08-27 | 2012-03-06 | The Invention Science Fund I, Llc | Health-related signaling via wearable items |
| US8125331B2 (en) | 2008-08-27 | 2012-02-28 | The Invention Science Fund I, Llc | Health-related signaling via wearable items |
| US20200214566A1 (en) | 2008-08-27 | 2020-07-09 | Gearbox Llc | Health-related signaling via wearable items |
| US8696917B2 (en) | 2009-02-09 | 2014-04-15 | Edwards Lifesciences Corporation | Analyte sensor and fabrication methods |
| CN101829396B (en) | 2009-03-27 | 2013-01-30 | 清华大学 | Micro-needle array chip and percutaneous administration patch using same and preparation method thereof |
| US20120037515A1 (en) | 2009-04-15 | 2012-02-16 | TheStateof Oregonactingbyand throughthestateBoard ofHigherEducationon behalf of thePortlandstateUniv | Impedimetric sensors using dielectric nanoparticles |
| US9351677B2 (en) | 2009-07-02 | 2016-05-31 | Dexcom, Inc. | Analyte sensor with increased reference capacity |
| US8490829B2 (en) | 2009-11-24 | 2013-07-23 | Pepsico, Inc. | Personalized beverage dispensing device |
| EP2343550B1 (en) | 2009-12-30 | 2013-11-06 | Imec | Improved microneedle |
| WO2011112742A2 (en) * | 2010-03-09 | 2011-09-15 | Biotectix, LLC | Electrically conductive and mechanically supportive materials for biomedical leads |
| US20130065257A1 (en) | 2010-03-16 | 2013-03-14 | Joseph Wang | Enzyme-logic biosensing |
| CN102958556B (en) | 2010-04-28 | 2017-02-15 | 金伯利-克拉克环球有限公司 | Nanopatterned medical device with enhanced cellular interaction |
| US10010272B2 (en) * | 2010-05-27 | 2018-07-03 | Profusa, Inc. | Tissue-integrating electronic apparatus |
| WO2011156095A2 (en) | 2010-06-10 | 2011-12-15 | The Regents Of The University Of California | Textile-based printable electrodes for electrochemical sensing |
| CN101915794B (en) | 2010-07-23 | 2013-07-24 | 浙江大学 | Preparation method of all-solid-state reference electrode |
| US20120172692A1 (en) | 2011-01-05 | 2012-07-05 | Janet Tamada | Sensing Fluid Concentration for Continuous Glucose Monitoring |
| US20130281808A1 (en) | 2012-04-20 | 2013-10-24 | National Cheng Kung University | Blood component detection device |
| US20130053660A1 (en) | 2011-04-22 | 2013-02-28 | Dar-Bin Shieh | Blood Component Detection Device |
| CN104114224A (en) | 2011-09-02 | 2014-10-22 | 加利福尼亚大学董事会 | Microneedle arrays for biosensing and drug delivery |
| TWM427950U (en) | 2011-09-23 | 2012-05-01 | Univ Nat Taipei Technology | Transdermal sensor |
| WO2013049381A1 (en) | 2011-09-28 | 2013-04-04 | Abbott Diabetes Care Inc. | Methods for analyte monitoring management and analyte measurement data management, and articles of manufacture related thereto |
| US8920628B2 (en) | 2012-11-02 | 2014-12-30 | Roche Diagnostics Operations, Inc. | Systems and methods for multiple analyte analysis |
| WO2014088492A1 (en) | 2012-12-07 | 2014-06-12 | Ascilion Ab | A microfabricated sensor and a method of sensing the level of a component in bodily fluid |
| US10426383B2 (en) | 2013-01-22 | 2019-10-01 | Medtronic Minimed, Inc. | Muting glucose sensor oxygen response and reducing electrode edge growth with pulsed current plating |
| WO2014152717A2 (en) | 2013-03-14 | 2014-09-25 | Sano Intelligence, Inc. | On-body microsensor for biomonitoring |
| WO2014160804A2 (en) | 2013-03-26 | 2014-10-02 | The Trustees Of Columbia University In The City Of New York | Fluid extraction and drug delivery system and methods using microneedles |
| US10004435B2 (en) | 2013-11-14 | 2018-06-26 | Dexcom, Inc. | Devices and methods for continuous analyte monitoring |
| WO2015143443A1 (en) | 2014-03-21 | 2015-09-24 | University Of Utah Research Foundation | Multi-site electrode arrays and methods of making the same |
| US20150276758A1 (en) | 2014-04-01 | 2015-10-01 | Anteneh Addisu | Biomarker Detection Device for Monitoring Peptide and Non-Peptide Markers |
| US9486169B1 (en) | 2014-04-18 | 2016-11-08 | Invoy Technologies, Llc | Ketone measurement system and related method with accuracy and reporting enhancement features |
| US10321858B2 (en) | 2014-08-18 | 2019-06-18 | Proteadx, Inc. | Apparatus and methods for transdermal sensing of analytes in interstitial fluid and associated data transmission systems |
| US9933387B1 (en) | 2014-09-07 | 2018-04-03 | Biolinq, Inc. | Miniaturized sub-nanoampere sensitivity low-noise potentiostat system |
| KR102426531B1 (en) | 2015-03-06 | 2022-07-29 | 삼성전자주식회사 | Biological information measuring device and manufacturing method thereof |
| US10028685B2 (en) | 2015-03-20 | 2018-07-24 | Stephen DeTurk | Device for determining fat expenditure from levels of ketone bodies that have passed through the skin and methods for determining the same |
| US20160296149A1 (en) | 2015-04-08 | 2016-10-13 | Sandia Corporation | In Vivo Extraction of Interstitial Fluid Using Hollow Microneedles |
| US11298039B2 (en) | 2015-04-17 | 2022-04-12 | Samsung Electronics Co., Ltd | Biometric information measuring sensor, biometric information measuring system, and method of measuring biometric information using the sensor |
| US20200254240A1 (en) | 2016-05-15 | 2020-08-13 | Biolinq, Inc. | Devices and Methods For The Incorporation Of A Microneedle Array Analyte-Selective Sensor Into An Infusion Set, Patch Pump, Or Automated Therapeutic Delivery System |
| US20210187286A1 (en) | 2016-05-15 | 2021-06-24 | Biolinq, Inc. | Devices and Methods For Low-Latency Analyte Quantification Enabled By Sensing In The Dermis |
| US20200101286A1 (en) | 2016-05-15 | 2020-04-02 | Biolinq, Inc. | Devices And Methods For The Generation Of Alerts Due To Rising Levels Of Circulating Ketone Bodies In Physiological Fluids |
| US20200297997A1 (en) | 2016-05-15 | 2020-09-24 | Biolinq, Inc. | Mechanical Coupling Of An Analyte-Selective Sensor And An Infusion System And Information Conveyance Between The Same |
| US10092207B1 (en) * | 2016-05-15 | 2018-10-09 | Biolinq, Inc. | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element |
| EP3523464B1 (en) | 2016-10-10 | 2024-04-24 | Biolinq Incorporated | Electro-deposited conducting polymers for the realization of solid-state reference electrodes for use in intracutaneous and subcutaneous analyte-selective sensors |
| US10827955B2 (en) | 2016-10-31 | 2020-11-10 | Dexcom, Inc. | Transcutaneous analyte sensor systems and methods |
| EP3592213B1 (en) | 2017-03-08 | 2023-03-22 | Abbott Diabetes Care Inc. | System for wellness and nutrition monitoring and management using analyte data |
| US11045142B1 (en) | 2017-04-29 | 2021-06-29 | Biolinq, Inc. | Heterogeneous integration of silicon-fabricated solid microneedle sensors and CMOS circuitry |
| USD875254S1 (en) | 2018-06-08 | 2020-02-11 | Biolinq, Inc. | Intradermal biosensor |
| EP4167853A4 (en) | 2020-06-17 | 2024-07-03 | Biolinq Inc | Devices and methods for application of microneedle arrays |
| DK4048152T3 (en) | 2020-07-29 | 2024-03-11 | Biolinq Incorporated | SYSTEM FOR CONTINUOUS ANALYTE MONITORING WITH MICRON NEEDLE ARRANGEMENT |
-
2017
- 2017-05-09 US US15/590,105 patent/US10092207B1/en active Active
-
2018
- 2018-10-04 US US16/152,372 patent/US10492708B1/en active Active
-
2019
- 2019-10-28 US US16/666,259 patent/US11406818B2/en active Active
-
2022
- 2022-06-27 US US17/850,832 patent/US20230137258A1/en active Pending
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12109032B1 (en) | 2017-03-11 | 2024-10-08 | Biolinq Incorporated | Methods for achieving an isolated electrical interface between an anterior surface of a microneedle structure and a posterior surface of a support structure |
| US11963796B1 (en) | 2017-04-29 | 2024-04-23 | Biolinq Incorporated | Heterogeneous integration of silicon-fabricated solid microneedle sensors and CMOS circuitry |
| US12011294B2 (en) | 2020-07-29 | 2024-06-18 | Biolinq Incorporated | Continuous analyte monitoring system with microneedle array |
| US11872055B2 (en) | 2020-07-29 | 2024-01-16 | Biolinq Incorporated | Continuous analyte monitoring system with microneedle array |
| US11857344B2 (en) | 2021-05-08 | 2024-01-02 | Biolinq Incorporated | Fault detection for microneedle array based continuous analyte monitoring device |
| US11904127B2 (en) | 2021-09-28 | 2024-02-20 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| US11986614B2 (en) | 2021-09-28 | 2024-05-21 | Biolinq Incorporated | Microneedle enclosure and applicator device for microneedle array based continuous analyte monitoring device |
| USD996999S1 (en) | 2021-11-16 | 2023-08-29 | Biolinq Incorporated | Wearable sensor |
| USD1033641S1 (en) | 2021-12-17 | 2024-07-02 | Biolinq Incorporated | Microneedle array sensor applicator device |
| USD1013544S1 (en) | 2022-04-29 | 2024-02-06 | Biolinq Incorporated | Wearable sensor |
| USD1038794S1 (en) | 2022-05-16 | 2024-08-13 | Biolinq Incorporated | Wearable sensor with illuminated display |
| USD1012744S1 (en) | 2022-05-16 | 2024-01-30 | Biolinq Incorporated | Wearable sensor with illuminated display |
| US12070313B2 (en) | 2022-07-05 | 2024-08-27 | Biolinq Incorporated | Sensor assembly of a microneedle array-based continuous analyte monitoring device |
| USD1035004S1 (en) | 2023-02-28 | 2024-07-09 | Biolinq Incorporated | Wearable sensor |
| USD1051745S1 (en) | 2023-04-27 | 2024-11-19 | Biolinq Incorporated | Wearable sensor |
Also Published As
| Publication number | Publication date |
|---|---|
| US10492708B1 (en) | 2019-12-03 |
| US10092207B1 (en) | 2018-10-09 |
| US20200085341A1 (en) | 2020-03-19 |
| US11406818B2 (en) | 2022-08-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20230137258A1 (en) | Tissue-penetrating electrochemical sensor featuring a co-electrodeposited thin film comprised of polymer and bio-recognition element | |
| Kim et al. | Continuous glucose monitoring using a microneedle array sensor coupled with a wireless signal transmitter | |
| Pappa et al. | Organic electronics for point-of-care metabolite monitoring | |
| US9309550B2 (en) | Analyte sensors having nanostructured electrodes and methods for making and using them | |
| Rozlosnik | New directions in medical biosensors employing poly (3, 4-ethylenedioxy thiophene) derivative-based electrodes | |
| Palmisano et al. | A disposable, reagentless, third-generation glucose biosensor based on overoxidized poly (pyrrole)/tetrathiafulvalene− tetracyanoquinodimethane composite | |
| Baluta et al. | Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor | |
| Chowdhury et al. | Highly sensitive electrochemical biosensor for glucose, DNA and protein using gold-polyaniline nanocomposites as a common matrix | |
| Soldà et al. | Glucose and lactate miniaturized biosensors for SECM-based high-spatial resolution analysis: a comparative study | |
| CN102112873B (en) | Composition for glucose sensing comprising of nanofibrous membrane and method for manufacturing non-enzymatic glucose biosensor using same | |
| Yang et al. | Glucose sensor using a microfabricated electrode and electropolymerized bilayer films | |
| Cuartero et al. | Polyurethane ionophore-based thin layer membranes for voltammetric ion activity sensing | |
| WO2021232109A1 (en) | Bioanalyte detection and monitoring | |
| US10480091B2 (en) | Sensors and methods of manufacture thereof | |
| Ates et al. | Carbon fiber microelectrodes electrocoated with polycarbazole and poly (carbazole-co-p-tolylsulfonyl pyrrole) films for the detection of dopamine in presence of ascorbic acid | |
| EP2043510A2 (en) | Analyte sensors and methods for making and using them | |
| Mariani et al. | Micro-and nano-devices for electrochemical sensing | |
| US20220257181A1 (en) | Minimally invasive continuous analyte monitoring for closed-loop treatment applications | |
| El-Maiss et al. | Mussel-inspired electro-cross-linking of enzymes for the development of biosensors | |
| O’Hare | Biosensors and sensor systems | |
| Liu et al. | Enzyme biosensors for point-of-care testing | |
| KR102290253B1 (en) | Bio sensor and manufacturing method thereof | |
| Mohammadzadeh Kakhki | Nafion based biosensors: A review of recent advances and applications | |
| Madden | Development and characterisation of macro-disc and micro-band electrodes for electrochemical sensing applications | |
| CN117761132B (en) | Phenylalanine sensing working electrode, electrode array, preparation method and application |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STPP | Information on status: patent application and granting procedure in general |
Free format text: DOCKETED NEW CASE - READY FOR EXAMINATION |