Specimen collection and handling
- 1. SPECIMEN COLLECTION AND HANDLING Symon Fidelis Nayupe BSc Medical Laboratory Sciences [Honors]
- 2. OBJECTIVES • Responsibility of specimen collection • How to collect and handle various specimens • Sample rejection
- 3. Introduction • Nurses often assume the responsibility of sample collection – wards • Proper sample collection and handling ensures quality and reliable lab results – sample degradation or compromise • Necessary for sample collectors to know and abide by proper sample collection and handling techniques
- 4. Specimen collection key issues • Consider differential diagnoses • Decide on test(s) to be conducted • Decide on clinical samples to be collected to conduct these tests • consultation between microbiologist, clinicians and epidemiologist
- 5. Specimen collection tubes 1. Ethylenediaminetetra-acetic acid [EDTA] •Purple top/ lavender top •Used for: routine haematology [FBC] Reticulocyte count Sickle test Glyco HB, Hb electrophoresis CD 4, thin smear, blood grouping •Not suitable for Calcium and coagulation •Invert several times soon after collection
- 6. Specimen collection tubes cont… 2. Red top [No clot activator] •As indicated, has no clot activator – plain •ALL BODY FLUIDS [CSF, Ascitic, Pleural, lymph aspirates etc] must be collected in this tube. •Invert several times soon after collection
- 7. Sample collection tubes cont… 3. Red top, with clot activator. •Used for chemistry, crossmatch, serology [VDRL, Hep B and C] •Invert several times after collection
- 8. Specimen collection tube cont.. 4. Blood culture bottles • Has bacterial growth medium and activated charcoal • Sample for blood culture is collected directly into BC bottle
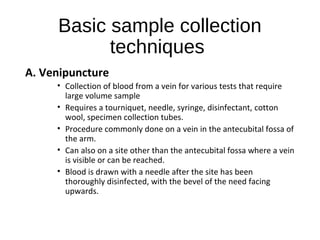
- 9. Basic sample collection techniques A. Venipuncture • Collection of blood from a vein for various tests that require large volume sample • Requires a tourniquet, needle, syringe, disinfectant, cotton wool, specimen collection tubes. • Procedure commonly done on a vein in the antecubital fossa of the arm. • Can also on a site other than the antecubital fossa where a vein is visible or can be reached. • Blood is drawn with a needle after the site has been thoroughly disinfected, with the bevel of the need facing upwards.
- 10. Blood samples – blood for smears Collection Capillary blood from finger prick • make smear • fix with methanol or other fixative Handling and transport Transport slides within 24 hours Do not refrigerate (can alter cell morphology)
- 11. Blood samples – blood for cultures Collection Venous blood • infants: 0.5 – 2 ml • children: 2 – 5 ml • adults: 5 – 10 ml Requires aseptic technique Collect within 10 minutes of fever • if suspect bacterial endocarditis: 3 sets of blood culture
- 12. Basic sample collection techniques • Urine collection • Wash hands • Clean meatus, female front to back • Start stream, then stop, collect specimen • Aseptic technique • Bedpan/mexican hat • To lab 15-20min post collection • Urine specimens • Random specimens, mid-stream urine, timed urine, sterile urine
- 13. Basic sample collection techniques • Stool specimens • Analysis of fecal material can detect pathological conditions ie: tumors, hemorrhage, infection • Tests: OB, ova and parasites, pus • Use a sterile container to collect stool. • Applicator sticks, direct collection, stool sample scoop. • Must reach the lab as soon as possible
- 14. Basic sample collection techniques • Vaginal or Urethral Discharge Specimens • Normally thin, nonpurulent, whitish or clear, small in amount • STD’s, UTI • Not Delegated • Assess external genitalia • If STD record sexual history • Physician’s order- vaginal/urethral
- 15. CSF • Collection • Lumbar puncture • Sterile tubes • Aseptic conditions • Trained person
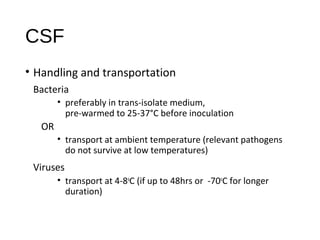
- 16. CSF • Handling and transportation Bacteria • preferably in trans-isolate medium, pre-warmed to 25-37°C before inoculation OR • transport at ambient temperature (relevant pathogens do not survive at low temperatures) Viruses • transport at 4-8o C (if up to 48hrs or -70o C for longer duration)
- 17. Common sample collection techniques – sputum Collection Instruct patient to take a deep breath and cough up sputum directly into a wide-mouth sterile container • avoid saliva or postnasal discharge • 1 ml minimum volume
- 18. Rejection of samples • All samples collected in wrong tubes are rejected • All samples untimely brought to the lab are rejected • Unlabeled samples are rejected • Insufficient samples are rejected • Compromised samples [haemolysed etc] are rejected • If a sample is rejected, the one rejecting it must indicate the reason for the rejection of the sample.
- 19. References • Lieseke CL, E Ziebig; Essentials of Medical Laboratory Practice, 1st Edi., FA Davis Company, Philadelphia, USA [2012]

![SPECIMEN
COLLECTION AND
HANDLING
Symon Fidelis Nayupe
BSc Medical Laboratory Sciences [Honors]](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-1-320.jpg)



![Specimen collection tubes
1. Ethylenediaminetetra-acetic acid [EDTA]
•Purple top/ lavender top
•Used for:
routine haematology [FBC]
Reticulocyte count
Sickle test
Glyco HB,
Hb electrophoresis
CD 4, thin smear, blood grouping
•Not suitable for Calcium and coagulation
•Invert several times soon after collection](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-5-320.jpg)
![Specimen collection tubes
cont…
2. Red top [No clot activator]
•As indicated, has no clot activator – plain
•ALL BODY FLUIDS [CSF, Ascitic, Pleural, lymph
aspirates etc] must be collected in this tube.
•Invert several times soon after collection](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-6-320.jpg)
![Sample collection tubes cont…
3. Red top, with clot activator.
•Used for chemistry, crossmatch, serology [VDRL,
Hep B and C]
•Invert several times after collection](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-7-320.jpg)








![Rejection of samples
• All samples collected in wrong tubes are rejected
• All samples untimely brought to the lab are
rejected
• Unlabeled samples are rejected
• Insufficient samples are rejected
• Compromised samples [haemolysed etc] are
rejected
• If a sample is rejected, the one rejecting it must
indicate the reason for the rejection of the sample.](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-18-320.jpg)
![References
• Lieseke CL, E Ziebig; Essentials of Medical
Laboratory Practice, 1st
Edi., FA Davis Company,
Philadelphia, USA [2012]](https://image.slidesharecdn.com/specimencollectionandhandling-150607195516-lva1-app6891/85/Specimen-collection-and-handling-19-320.jpg)