Abstract
Glabridin, a prenylated isoflavonoid of G. glabra L. roots (European licorice, Fabaceae), has been associated with a wide range of biological properties such as antioxidant, anti-inflammatory, anti-atherogenic, regulation of energy metabolism, estrogenic, neuroprotective, anti-osteoporotic, skin-whitening. While glabridin is one of the most studied licorice flavonoids, a comprehensive literature survey linked to its numerous bioactivities is unavailable. The present review provides a comprehensive description of glabridin as a key chemical and biological marker of G. glabra, by covering both its phytochemical characterization and reported biological activities.
Both glabridin and standardized licorice extracts have significant impact on food, dietary supplements (DSs) and cosmetic markets, as evidenced by the amount of available patents and scientific articles since 1976, when glabridin was first described. Nevertheless, a thorough literature survey also reveals that information about the isolation and chemical characterization of this important marker is scattered and less detailed than expected. Accordingly, the first part of this review gathers all analytical and spectroscopic data required for the comprehensive phytochemical characterization of glabridin. The four most frequently described and most relevant bioactivities of glabridin are its anti-inflammatory, anti-atherogenic, estrogenic-like effects, and its capacity to regulate energy metabolism. While all bioactivities reported for glabridin belong to a wide array of targets, its principal biological properties are likely interconnected. To this end, the current state of the literature suggests that the biological activity of glabridin mainly results from its capacity to down-regulate intracellular reactive oxygen species, bind to antioxidant effectors, and act on estrogen receptors, potentially as a plant-based Selective Estrogen Receptor Modulator (phytoSERM).
Keywords: Glabridin, Chemical Characterization, Anti-Atherogenic, PhytoSERM, Metabolic Syndrome, Pharmacokinetics
1 Introduction
Glabridin (Glab) is an isoflavonoid originally isolated from the roots of Glycyrrhiza glabra L. (Fabaceae). Since its first isolation and characterization in 1976, the number of publications dealing with its chemical and biological characterization had increased exponentially (Figure 1A). In 2011 and 2012, an average of 14 articles per year was dedicated to the study of Glab, and most of them focused on its biological activities. Glab is widely considered to be a phytoestrogen and has been associated with numerous biological properties ranging from antioxidant, anti-inflammatory, neuroprotective, anti-atherogenic effects, to the regulation of energy metabolism, but also including anti-tumorigenic, anti-nephritic, antibacterial and skin-whitening activities (Figure 1B).
Figure 1. Scientific Literature, Domains of Investigations and Type of Patents Associated with Glabridin since 1976.
Panel A represents the occurrence of the scientific literature (articles, reviews, reports, except patents) on Glab including its phytochemical analyses and the evaluation of its biological activities. The number of publications dedicated to Glab increases exponentially, reaching a maximum of 14 annual articles published in the past two years. Panel B highlights the distribution of the literature in the different domains of investigation. Most of the scientific literature has been dedicated to the evaluation of Glab’s bioactivities (60%). Among them, 23% are associated to its anti-atherogenic properties (Panel B, area A), and 15% are linked to its capacity to regulate energy metabolism (Panel B, area B). A share of 30% of the biological investigations (Panel B, area C) is almost equally divided between the evaluation of its estrogenic, anti-osteoporotic and anti-inflammatory activities. Panel C details the focus area of patents associated to Glab, its isolation, chemical modification and applications. Cosmetic and dermatologic patents represent 70% of all issued patents, and are mostly associated with diverse formulations and applications of G. glabra roots extract enriched in Glab.
The term “Glabridin-40” is listed in the International Nomenclature of Cosmetic Ingredients (INCI) as Glycyrrhiza glabra (root) extract enriched in Glab. Those enriched extracts are widely used in cosmetic formulation as anti-inflammatory, antioxidant and skin whitening agents. Approximately 166 patents are associated with the isolation, synthesis, formulation and applications of Glab. Among them, 82% were Chinese or Japanese patents, and 70% had a cosmetic or dermatologic application. To date, the number of patents and research articles linked to Glab study are almost equal (Figure 1C).
Glab and, to a lesser extent, extracts enriched in Glab, have a growing impact not only on cosmetics but also on the food and dietary supplements (DSs) market. This observation can be illustrated by the recent development of Glavonoid®, an extract enriched in Glab, as a Novel Food ingredient [1], or the inclusion of Glab in formulations used for muscle building [2], hangover alleviation [3], and even health care chocolate [4]. Considered to be a phytoestrogen, Glab is also an interesting phytonutrient with beneficial effects such as cardiovascular protection [5] and bodyweight control [6].
The principal objective of this review is to draw a comprehensive chemical and biological profile of Glab. Methods of extraction and enrichment, isolation, structural characterization, chemical modification and quantitative analysis are presented in the first part of this article. Pharmacological properties and pharmacokinetic (PK) parameters reported for Glab are covered in the second part so as to exhaustively describe its biological fingerprint. The meta-analysis of Glab’s bioactivities suggests the existence of a connection between its major reported biological properties, and reveals the question of their physiological relevance in terms of achievable active concentrations in body fluids and tissues. Finally, this survey aims at a better understanding of Glab and its biological mode of action, and addresses the relevance of future investigations so as to unravel the pharmacological and pharmacokinetic profile of Glab. Altogether, the present article provides a comprehensive description of Glab as a key chemical and biological marker from the roots of G. glabra.
2 Phytochemical characterization of glabridin
2.1 Occurrence and biosynthesis
Natural isoflavonoids are mainly distributed in leguminous plants (Fabaceae) and play ecophysiological roles as defensive agents against other organisms ranging from animals to microbes (phytoalexins). Some isoflavonoids are considered symbiotic signals to rhizobial bacteria to form nitrogen-fixing root nodules [7, 8]. The isoflavans (syn. deoxyisoflavanones) make up a unique subclass considering that the heterocycle ring C does not contain a double bond between carbon 2 and 3, nor a carbonyl group (Figure 2). Therefore, no conjugated double bonds exist between the rings A and B. Isoflavonoids possessing pterocarpan and isoflavan skeletons are the most frequently found phytoalexins of leguminous plants [9, 10].
Figure 2. Overview of Sub-classes of Isoflavonoids and their Biogenetic Relationship with Glabridin.
(A) Isoflavans such as vestitol have been demonstrated to be formed through the enzymatic reduction (pterocarpan reductase, PTR) of their pterocarpan precursors such as medicarpin. The dihydrofuran ring cleavage explains the origin of the hydroxyl attached to C-2′ (in red), and present in most isoflavans [11]. (A and B) Isoflavans and pterocarpans are generally among the end products of the flavonoids biosynthetic pathway, and are formed through at least four enzymatic steps, symbolized by the arrows starting from the corresponding isoflavone. (C) A pterocarpan prenyltransferase (GmG4DT), identified in Soybean was demonstrated to be responsible for the prenylation on C-8 (in purple) of pterocarpans [13]. Considering the known enzymatic activity of PTR, shinpterocarpin, also found in the roots of G. glabra [12], can be considered as the putative biosynthetic precursor of Glab.
The biosynthesis of these two subclasses of isoflavonoids is considered to be closely related, because most, if not all, isoflavans bear a hydroxyl group at C-2′ in the B-ring, representing the functional group which originally participated in the ether linkage to C-4 to produce the pterocarpan skeleton. More precisely, as demonstrated for medicarpin and vestitol, isoflavan derivatives are the end products of pterocarpan reductase enzymes (PTR) [11] (Figure 2A). To date, the specific biosynthetic pathway that leads to Glab and its precursor remains unknown. However, PTR may also be present in the genus Glycyrrhiza and, therefore, the bioprecursor of Glab could be a pterocarpan. Based on its characteristic features, shinpterocarpin, which has also been described in licorice [12], could be considered as the immediate pterocarpan precursor of Glab. Additionally, a pterocarpan specific prenyltransferase (GmG4DT) has been identified in Soybean (Glycine max, also Fabaceae) [13]. Both shinpterocarpin and glabridin share a prenyl function linked to C-8 of the A ring, suggesting that shinpterocarpin could stem from desmethylmedicarpin and be formed through GmG4DT prenyltransferase activity (Figure 2B and 2C).
Interestingly, Glab has been demonstrated to be localized only in the cork layer and the decayed part of the roots of G. glabra [14], representing 0.08 to 0.35% of the roots’ dry weight [15]. In 1978, Glab was identified as a species-specific secondary metabolite of G. glabra, and this designation was later confirmed by Kondo et al. through the comparative analysis of genetic and chemical markers from the three principal licorice species: G. uralensis Fisch. ex DC., G. glabra L., and G. inflata Batalin [16].
Considering the established role of Glab as a phytoalexin, its quantity in the investigated material may reflect the environmental stress to which producing parts of G. glabra were exposed. Accordingly, Glab content may fluctuate between samples depending on the type of cultivation and the geographical area from which the roots were harvested.
2.2 Methods for G. glabra extraction and enrichment in glabridin
Glab is generally not consumed as a single chemical entity but rather as constituent of G. glabra crude extract. This reveals the importance of method development dedicated to the extraction of G. glabra roots and the enrichment in Glab. Most licorice extracts are produced for commercialization in the cosmetic, food or DSs markets, hence requiring the use of non-toxic solvents and environmentally safe methodologies. Extraction with ethanol (EtOH) [17], aqueous EtOH [18], supercritical fluid CO2 [19], and more recently, imidazolium based ionic liquids (ILs) [20] are preferred for the production of G. glabra extracts enriched in Glab. Other enrichment methods resort to the use of diverse macroporous resins [21, 22].
A licorice flavonoid oil (LFO) has been prepared by extracting a G. glabra ethanolic extract (EtOH 95%, 5 volumes/1volume of roots) with medium-chain triglycerides (MCT, C8:C10 = 99:1) [17]. Accordingly, LFO represents the hydrophobic polyphenol-rich fraction of licorice ethanol extract. Standardized to a Glab concentration of 1% (w/w), the oily LFO product is commercialized under the name Glavonoid®, which has been recently accepted by the European Food Safety Authority as a Novel Food Ingredient [1].
A supercritical fluid extraction method of G. glabra roots using CO2 and co-solvents such as MeOH or EtOH has been developed by Cho et al. [19]. The optimum conditions were found to be an extraction over 3 hours at 80 °C, 420 bar, and with 25% MeOH as co-solvent (which was demonstrated to be more effective than EtOH for the enrichment of Glab). The final Glab concentration in the supercritical CO2 extract was not indicated in this study. Another supercritical fluid extract (LSC) was prepared by Ahn et al. [23] for pharmacological studies. Briefly, 154 g of roots were extracted during 1 hour, at 300 bar and 40 °C with a CO2 flow rate of 150 g/min. The extraction yield was defined as 3.57% w/w, and one gram of LSC extract contained 45.12 ± 0.14 mg of Glab (4.5% w/w).
GutGuard™ is a G. glabra extract developed by Natural Remedies, Pvt., Ltd., in Bangalore, is reported to be standardized in Glab (≥ 3.5% w/w), glabrol, and caffeic acid derivatives, and has a total flavonoid content of ≥ 10.5% w/w. However, the methods of extraction and further preparation of GutGuard have not been disclosed [24, 25].
Tian et al. [18] have analyzed the parameters enabling a concomitant extraction and enrichment of both glycyrrhizic acid and Glab. Different solvents (EtOH, MeOH, dichloromethane) at various temperatures and varying durations were tested and led to the conclusion that a mixture of EtOH:H2O (30:70 v/v) and a dipping time of 60 min at 50 °C represented the optimum conditions. The same authors also compared the capacity of various solvents (Chloroform (CHCl3), n-hexanes, H2O, H2O/MeOH and MeOH) to extract glycyrrhizic acid, Glab and liquiritin simultaneously. Pure MeOH turned out to be the best extraction solvent when used in a ratio of 1:70 (weight of plant material [g] per volume of solvent [mL]) during 120 min [26]. Nevertheless, considering general safety concerns of residual MeOH in extracts, extraction with EtOH should be preferred for commercial products.
ILs are attractive “green” and functional solvents in the field of organic synthesis and in various separation and extraction processes of organic acids or phenolic compounds. Li et al. [20] have developed specific ILs in order to selectively extract Glab from its natural matrix and suggested that this type of “green” extraction could be used as a first efficient step for the purification of Glab. A series of 1-alkyl-3-methylimidazolium (ILs) with different anions and alkyl chain lengths of cations were selected for this purpose.
A solid phase extraction (SPE) method using Molecular Imprinted Polymers (MIPs) has been proposed as a first enrichment step for the analysis of Glab content in different types of matrices [21]. For this purpose, the crude extracts of G. glabra or other herbal mixtures were washed from the MIPs using a mixture of MeOH:H2O (60:40 v/v). In the second step, the Glab retained on the polymer was eluted with acetonitrile (MeCN) containing 0.5% of acid trifluoroacetic (TFA). MIPs were claimed to be selective for conducting targeted analyte isolation. However, the reported Glab recovery was only 42 to 56%, and Glab was also found to be present in the washing fraction. Subsequently, Xu et al. [22] have developed an enrichment method by studying the adsorption and desorption properties of Glab on different macroporous resins including HPD100, HPD300, HPD800, NKA and H103. The content of Glab in the final enriched fraction, after using HPD100, was ~150-fold higher than in the starting crude extract. One major drawback of these enrichment techniques is the necessity to employ a solid phase interaction step, which is known to lead to potential irreversible adsorption. For example, the recovery of Glab after passage through HPD100 was 80%, indicating that 20% of Glab remains on the resin. On the other hand, the cited SPE techniques, using MIPs or macroporous resins, are well suited for the enrichment and subsequent isolation of Glab.
2.3 Isolation from its natural source/purification processes
Most of the techniques reported for the isolation of Glab require a minimum of three chromatographic steps, including at least one step of liquid/liquid (L/L) partition and two steps of silica gel chromatography with the frequent use of benzene/ether as final eluent [27, 32] (Figure 3). Considering the chemical complexity of G. Glabra roots, it is understandable that purifying this prenylated isoflavan in less than three to four chromatographic steps remains a challenging task. In addition to silica gel, the second type of stationary phase which has been frequently used was polyamide, although both materials are known for unwanted properties such as irreversible adsorption and chemical modification through oxidation. In summary, Saito et al. [29] were able to isolate Glab after two steps of silica gel chromatography and one final purification on polyamide using MeOH as eluent. After L/L partition of the crude extract, Vaya et al. [28] isolated Glab from the organic layer, following one step of aluminum oxide chromatography and two steps of silica gel flash chromatography. Like Mitscher et al. [31], Cui et al. [30] have reported a procedure involving only two steps of silica gel chromatography from the CHCl3 fraction obtained by L/L partition, and one final step of crystallization. A method that combined SPE, to obtain an enriched fraction, and preparative HPLC has been developed by Jiao et al. [33]. Glab was also isolated in our laboratory, after three steps of Medium Pressure Liquid Chromatography (MPLC) on HW-40F, Sephadex LH-20 and a final purification step on a cyano-derivatized silica adsorbent (LiChroCN).
Figure 3. Various Purification Methods Reported for Glabridin since its Original Isolation.
Different methods have been reported throughout the years for the purification of Glab. All of them comprise of four different methods and share an initial liquid/liquid partition step. At all four levels, silica gel chromatography remains widely used, using different types of organic solvents with benzene/ether being a preferred eluent. Gel filtration with the sorbents HW-40F or Sephadex LH-20 has not been commonly reported. Solid phase chromatography on polyamide has been primarily used in patented methods. The yield of Glab isolation fluctuates between studies, and the final purity of the products is seldom indicated. Nd stands for not disclosed. *In the study of Vaya et al. [28], the purity of each isolated compounds was checked by HPLC and GC-MS, but the final purity percentage of Glab was not reported.
Numerous methods for the isolation of Glab have been patented. Most of them comprised four steps including two chromatographic steps in order to obtain a reported pure Glab. These patented methods follow the same processes as those described above. Most require the use of a polyamide column during the purification process [34, 38]. The literature survey also revealed that the purity of Glab was determined exclusively by HPLC-UV [28, 30, 39], and that with the exception of some patents, the purity of isolated Glab and/or Glab used for bioassays remained undisclosed.
Because all reports involve multiple chromatographic steps to obtain pure Glab from its natural source, it can be concluded that the purification processes remain complicated, time-consuming, and require large amounts of volatile organic solvents. Additionally, most procedures are hardly suited for large scale production of Glab, and experimental details provided in the reports are frequently insufficient to ensure reproducibility.
2.4 Structural characterization
Naturally occurring Glab was isolated and characterized for the first time in 1976 by Saito et al [29]. The authors determined the configuration of carbon C-3 to be R by optical rotary dispersion according to the positive Cotton Effect observed between 260 and 300 nm.
To date, the parameters required for an exhaustive chemical characterization of Glab are spread throughout the literature. Nuclear Magnetic Resonance (NMR) data of Glab were mostly acquired in deuterated chloroform (CDCl3) [27] and dimethyl sulfoxide (DMSO-d6), at 300 or 400 MHz [40]. Both solvents offer good signal dispersion, and spectra acquired in DMSO-d6 are essentially free of resonances overlap when using 300–600 MHz instruments. The 1H NMR spectra of Glab acquired in these solvents are not identical (Table 1, Appendices A and B), especially in the aromatic region, where the signals of protons H-6 and H-5′ are overlapping in CDCl3. In some articles, the proton assignments have been inaccurate, particularly with regard to the aromatic protons which have been reported as signal ranges (e.g., “6.37–6.41 (2H, m)”) [28, 30].
Table 1.
1H and 13C NMR Data of Glabridin in DMSO-d6 and in CDCl3 (600 and 225 MHz).
| in DMSO-d6± | In CDCl3± | ||||
|---|---|---|---|---|---|
|
| |||||
| Position | mult. | δc (225 MHz) | δH (J in Hz) | δc (225 MHz) | δH (J in Hz) |
|
| |||||
| 2 pro S | CH2 | 69.734 | 3.929, t (10.27) | 69.942 | 4.007 t (10.56) |
| 2 pro R | 4.228, td (10.27, 2.61) | 4.371 td (10.56, 2.55) | |||
| 3 | CH | 30.883 | 3.287, dddd | 31.625 | 3.481, dddd |
| 4 pro S | CH2 | 29.985 | 2.694, ddd (15.52, 4.77, 1.76) | 30.568 | 2.860 ddd (15.62, 4.71, 1.57) |
| 4 pro R | 2.891, dd (15.52, 11.36) | 2.971 dd (15.62, 10.77) | |||
| 5 | CH | 129.222 | 6.827, d (8.21) | 128.934 | 6.827, d (8.23) |
| 6 | CH | 108.085 | 6.283, d (8.21) | 108.691 | 6.370, d (8.23) |
| 7 | C | 151.211 | 151.873 | ||
| 8 | C | 109.055 | 109.888 | ||
| 9 | C | 149.237 | 149.692 | ||
| 10 | C | 114.748 | 114.231 | ||
| 1′ | C | 117.416 | 119.980 | ||
| 2′ (OH) | C | 155.848 | 9.384, s | 154.310 | |
| 3′ | CH | 102.457 | 6.318, d (2.42) | 103.040 | 6.311, d (2.30) |
| 4′ (OH) | C | 156.871 | 9.109, s | 155.141 | |
| 5′ | CH | 106.266 | 6.179, dd (8.32, 2.42) | 107.974 | 6.388, dd (8.37, 2.30) |
| 6′ | CH | 127.559 | 6.857, d (8.32) | 128.397 | 6.955, d (8.37) |
| 2″ | C | 75.225 | 76.855* | ||
| 3″ | CH | 129.339 | 5.644, d (9.89) | 129.146 | 5.562, d (9.90) |
| 4″ | CH | 116.416 | 6.535, d (9.89) | 116.90 | 6.646, d (9.90) |
| 5″ | CH3 | 27.221 | 1.341, s | 27.762 | 1.410, s |
| 6″ | CH3 | 27.350 | 1.336, s | 27.516 | 1.428, s |
Data were acquired in our laboratory under quantitative conditions at 298 K using a 90° single-pulse experiment (DE = 39.71 μsec, D1 = 60.00 sec, P1 = 8 μsec for DMSO-d6 and 10.25 μsec for CDCl3, ds =4, ns = 32) on a Bruker AVANCE 600.13 MHz equipped with a 5 mm TXI cryoprobe and a Bruker Avance 900 MHz. Off-line data processing was performed using Mnova NMR software package. The 1H chemical shifts (δ) were expressed in ppm with reference to the residual solvent signal (DMSO-d5, 2.500 ppm and CDCl3, 7.250 ppm relative to the TMS scale), and coupling constants (J) were given in Hertz. In CDCl3 at 300, 400, and 600 MHz, resonances of the protons H-5′ and H-6 are overlapping (bold font). 13C and 1H NMR data in DMSO-d6 and CDCl3 acquired at 225 MHz and 600 MHz, receptively were in accordance with previous published work from Jirawattanapong et al. [40] and Kinoshita et al. [27] (see Appendices B and A).
13C signal below the solvent signals.
An X-ray analysis of Glab, crystallized from MeOH, was published recently [41]. The crystalline sample obtained in MeOH was defined as orthorhombic P212121 (Z = 4). The absolute configuration of Glab was assumed to be the same as deduced from previous studies. A circular dichroism (CD) spectrum was recorded by us from an enantio-pure crystalline sample and found to be in accordance with data acquired by Kim et al. [42] (Figure 4). These authors had also determined the absolute configuration of synthetic Glab enantiomers isolated by chiral chromatography using CD polarimetry. The same authors assigned the coupling constants of H-3 which was determined to be a dddd resonance with JH-2eq, 3 = 3.7 Hz, JH-2ax,3 = 10.5 Hz, JH3,4ax = 10.5 Hz, and JH3,4eq = 5.5 Hz) after a simulation of the 1H NMR spectrum acquired in CDCl3 and use of the ACDLABS HNMR viewer program. The 1D 1H and 13C NMR data of Glab in DMSO-d6 and CDCl3 at 600 MHz and 225 MHz have also been recorded in our lab and led to unequivocal assignments for all proton and carbon resonances (Table 1, Figure 4, Appendices A and B).
Figure 4. Chemical Characterization of Glabridin (1D qNMR, UV and CD spectra).
Panels A and B represent the 1D, 1H and 13C (DEPT-Q) NMR spectra of Glab in DMSOd6, which were acquired by us. This solvent avoids overlap of the NMR resonances for most modern high-fields magnets. Precise 1H and 13C chemical shifts corresponding to this figure are available in Table 1 and were determined according to HMBC and HSQC correlations. 1H and 13C chemical shifts of Glab in DMSO-d6 at 300 MHz and in CDCl3 at 400 MHz are available in Appendix A. The comparative 1H NMR spectra in DMSO-d6 and CDCl3 at 900 MHz is available in Appendix B. Panel C shows the CD spectrum of enantio-pure 3R-glabridin obtained from a crystal sample and in accordance with previous data [42]. Panel D gives the characteristic UV spectrum of this prenylated isoflavan also acquired in acetonitrile. The Infra-Red (IR) spectrum is available in Appendix C. HPTLC profiles of Glab and G. glabra crude extracts are in Appendix D.
There is a demand for enantio-pure Glab, both as a chemical reference standard for quality control and identification of G. glabra materials and for biological/pharmacological investigations. As structurally related isoflavans are required for structure activity relationship (SAR) studies, methods for chemical synthesis of Glab and other structurally related isoflavans have been developed. This topic will be addressed in the following.
2.5 Chemical synthesis of glabridin and isoflavan derivatives
Frequently, chemical synthesis is considered an efficient way to obtain quickly a large amount of pure reference material. However, in the case of Glab containing a stereogenic center on carbon 3, achiral chemical synthesis will inevitably lead to the production of an enantiomeric mixture. In 2007, Yoo and Nahm have proposed a rapid and efficient synthesis of racemic Glab in four steps and with 85% yield [43, 44]. Two years later, Jirawattanapong et al. [40] published an article on the synthesis of Glab derivatives (3″, 4″-dihydro- and 2′, 4′-diesters derivatives) starting from isolated Glab. A patent has been issued for the synthesis of Glab and other isoflavans derivatives as PPAR-γ ligands [45]. Optical resolution of these synthetic isoflavans was achieved through chiral chromatography using optically active polysaccharides and amino acids [46]. Pure enantiomers from the synthetic Glab were also isolated by chiral chromatography (Table 2), and their absolute configuration was determined by CD [42]. In summary, while synthetic methods for enantiopure Glab have been described, they involve relatively elaborate protocols due to the need for chiral separation and, therefore, retain the preparative chromatographic steps otherwise required for ab initio purification of Glab from its natural source.
Table 2.
HPLC Methods for Analysis and Quantitation of Glabridin
| Type of analysis | Columns-gradient - detection conditions | Rt.1(min) | Ref. |
|---|---|---|---|
|
| |||
| Enriched fraction in glabridin (35.2% w/w) | Symmetry C18 (300 × 19 mm, 15 μm) MeOH/H2O gradient: 70% MeOH during 40 min to 100% MeOH in 2 min during 20 min Flow rate: 10 mL/min Detection UV: 283 nm |
35–37 min | [33] |
|
| |||
| Isolated glabridin | Diamodsil™ C18 (250 × 4.6 mm, 5 μm) MeCN/H2O (0.05% TFA) gradient: 60% MeCN during 20 min to 85% in 2 min and during 15 min Flow rate:1 mL/ min |
12.5 min | [33] |
| LC-MS/MS Parameters (Ion trap MS ESI source) | MeCN/H2O (2% FA): 70% MeCN, isocratic mode Flow rate: 0.4 mL/min Capillary temperature: 250 °C Sheath gas: 0.8 L/min, auxiliary gas: 0.10 L/min Electrospray voltage: 4 kV, Capillary voltage: 32 V Tube lens offset: −5V, relative collision energy 30% |
Not indicated | |
|
| |||
| Glabridin enantiomers | Sumichiral OA-7000 (20 × 25 mm; 5 μm) MeCN/H2O (0.1% TFA) gradient: 50% MeCN for 3 min to 70% MeCN in 10 min Flow rate: not indicated Detection UV |
3R:~7.4 3S: ~8 min |
[42] |
|
| |||
| Polyherbal mixture after extraction (30% EtOH) | Licrosphere 100 RP18e (250 × 4.6 mm, 5 μm) MeCN/H2O gradient: 50% MeCN to 80% in 20 min Flow rate: 1 mL/min Detection UV: 230 nm |
14.9 min | [58] |
|
| |||
| Glabridin and its derivatives GDA, GDH | Hypersil-Keystone C18 column (250 × 4.6 mm, 5 μm) MeCN/H2O: 76% MeCN during 9 min to 90% in 9 min and during 12 min Flow rate: 1 mL/min Detection UV: 280 nm |
4.7 min 7.8 min (GDA) 25.0 min (GDH) |
[54] |
|
| |||
| Blood samples containing glabridin -after SPE- | LUNA 5μ C18 (2.0 mm × 150 mm, 5 μm) 85% MeOH in H2O (0.1% FA) isocratic mode Flow rate: 0.2 mL/min |
[59] | |
| LC-MS/MS Parameters: (Turbo ionspray tandem MS) | Negative ionization mode Ion spray voltage: −4000 V Heater gas (air) temperature: 600 °C, 80 psig Nebulizing gas (air): 50 psig, Curtain gas (N2): 40 psig Multiplier voltage: 2000 V Multiple reaction monitoring (MRM): m/z 323.1→201.3 Collision energy: −32 eV |
4.2 min | |
Rt: Retention time of Glab under the described conditions. The HPTLC profiles of crude extracts from the roots G. glabra are in Appendix D.
2.6 Glabridin stability: prodrugs and formulation
Ao et al. [47] have evaluated various factors influencing Glab stability, such as temperature, pH, light exposure, and humidity. While Glab was found to be unstable under basic conditions (pH > 7), it remained stable at neutral pH. Glab was stable between 4 and 60 °C, and started to degrade only at temperatures above 60 °C. Moreover, degradation was shown to occur at room temperature under natural light. It was finally concluded that, for long term storage, Glab has to be kept in a dry, dark, and low oxygen environment. The structural identity of degradation products remains yet unknown.
In order to enhance Glab solubility in aqueous media, but also to increase its stability in formulations, two different techniques have been developed. One approach consists of synthetizing 2′,4′-diester or -diether derivatives, the second utilizes a physical protection of Glab through encapsulation. Ether or ester derivatives of Glab can be considered as pro-drugs, especially when taking into account that 2′,4′-dimethoxyglabridin undergoes demethylation in vivo, as indicated by Rosenblat et al. [48]. Glabridin 2′,4′-O-di-β-D-glucopyranoside was synthetized as UVB absorber for sunscreen formulation [49], as tyrosinase inhibitor for a skin whitening formulation [50], and as anti-irritant [51]. The 2′,4′-dihydroxyl functions can also be protected through an etherification with an alkyl residue [52], or by esterification with undecylenic acid. The resulting hydrophobic derivatives have been also claimed to increase the skin adsorption of Glab [53, 54].
A methodology for Glab encapsulation in nanoemulsions has been developed by Hsieh et al. [55]. Such nanoemulsions might have the potential to be readily absorbed by the skin, and easy to sterilize by filtration. The optimum conditions for preparing Glab nanoemulsions were found to consist in an emulsifier (Tween 80 + Span 80) concentration of 5.3%, a caprylic triglycerides concentration of 3.65%, with a homogenization pressure of 129 MPa. Other methodologies of encapsulation include the development of a chitosan nano-complex, [56] and the development of microsponges formed by emulsion solvent evaporation [57]. Both methods have been demonstrated to enhance Glab stability in topical formulations. In the chitosan nano-complex, the hydroxyl functions of encapsulated Glab were proposed to interact with the amino groups of chitosan, and the maximum encapsulation efficiency was reached when using 84% of N-propionic chitosan for 1% of Glab in the formulation [56]. In contrast, Glab was shown to be incorporated passively into the pores of the microsponges, and no specific physical interactions were observed [57].
2.7 Analytical studies and quantitation
Reproducible and precise analytical methods are needed for the quantitation of Glab in standardized G. glabra extracts, or in biological samples for the subsequent determination of pharmacokinetic (PK) parameters. Accurate methods are also required for the quality control of polyherbal mixtures containing licorice, and when performing Glycyrrhiza species identification (Table 2). In summary of the available literature, quantitation of Glab in different matrices has been carried out by HPLC following UV (230 or 280 nm) [54, 58] or MS detection [59], and recently by HPTLC [60] (Appendix D for HPTLC profiles). Except for the chiral separation of Glab enantiomers [42], most of the HPLC analyses have utilized reverse phase C18 columns [54, [58, 59]. Some of the principal studies are detailed below.
Kamal et al. [58] have developed an HPLC method for the quantitation of Glab from polyherbal preparations and crude extracts. Both were analyzed after extraction with 30% aqueous ethanol (overnight soaking followed by extraction under reflux during 45 min) on a reverse phase C18 (Merck, LiChrosphere 100 RP18e, 250 × 4.6 mm, 5 μm) column with an MeCN/H2O gradient from 50 to 80% MeCN at 1 mL/min. Under these conditions, Glab was detected at 230 nm with a retention time (Rt) of 14.90 ± 0.02 min. The Glab enantiomers could be separated on a chiral Sumichiral OA-7000 (Sumika Chemicals, Osaka, Japan) column, a stationary phase composed of derivatized beta-cyclodextrins [42]. Aoki et al. validated an analytical method for the determination of Glab in plasma after SPE using C8 cartridge, followed by an LC-MS/MS analysis and using mefenamic acid as internal standard [59]. Ester derivatives of Glab (glabridin diacetate [GDA] and dihexonate [GDH]), used in topical formulations, have been quantified by HPLC-UV (280 nm) on a reverse phase C18 column (250 × 4.6 mm, 5 μm; Thermo Hypersil-Keystone, Bellefonte, PA, USA), eluted with a mixture of MeCN/H2O as follows: 76% during 9 min to 90% MeCN in 9 min and during 12 min at a flow rate of 1 mL/min [54]. Under these conditions, the Rt of Glab was 4.70 min, and the esters eluted at 7.76 and 25.04 min for GDA and GDH, respectively.
3 Reported Biological Activities
Glab has been demonstrated to have anti-inflammatory properties, both in vitro and in vivo, which will positively influence several of its other bioactivities (anti-atherogenic, neuro-protective, anti-osteoporotic). The most studied and documented beneficial effect of Glab is its capacity to regulate intracellular antioxidant systems and prevent the development of atherosclerotic lesions. Another key mechanism behind Glab’s health benefits is its ability to bind to the human estrogen receptor (hER) and to act potentially as a phytoSERM [61]. Like many phytoestrogens, Glab participates in the regulation of cellular energy expenditure, displaying potential for the prevention of metabolic syndrome [62]. Other reported properties of Glab are somewhat less documented and include its anticancer, antinephritic, and antibacterial activities as well as its capacity to inhibit melanogenesis.
3.1 Anti-inflammatory properties
Inflammation is the starting point for many chronic diseases including cardiovascular, degenerative diseases and cancer. Multiple factors (cytokines, prostaglandins PGs), enzymes (COX-2, iNOS), and nuclear factors (NF-κB, AP-1) are involved in the expression of inflammation. The inducible enzymes, cyclooxygenase-2 (COX-2), and nitric oxide synthase (iNOS) are involved in the production of PGs (e.g. PGE2), thromboxanes (TXs) and NO, respectively. In macrophages, expression of iNOS-related genes is mainly regulated at the transcriptional level by regulators belonging to the (NF-κB)/Rel family of transcription factors. The latter also regulate the production of other inflammatory cytokines such as tumor necrosis factor-α (TNF-α) or interleukines (ILs; e.g., IL-1β, IL-6). Overproduction and cellular release of all these inflammatory mediators (PGE2, NO, TNF-α, ILs) are responsible for the characteristic expression of inflammation and its subsequent side effects.
The anti-inflammatory effect of Glab was first demonstrated in 1998 by Yokota et al. [63]. At the concentration of 6.25 μg/mL, Glab inhibited 31.9% of COX activity stimulated by the addition of arachidonic acid (AA). Since this discovery, the anti-inflammatory properties of Glab have been evaluated in vitro on murine macrophages [64], microglia [65], promyelocytic [64], and dendritic cells [66], as well as in vivo on BALB/c mice [67] and BDF1 mice [68], which suffered from colonic inflammation or sepsis (Table 3).
Table 3.
Principal Bio-Activities Reported for Glabridin
| Reported Activities-Targets | Bio. Systems1 | Active2 Conc. | Ref. | |
|---|---|---|---|---|
| Anti-inflammatory | Inhibition of 31.9% of COX activity stimulated By arachidonic acid | extracted COX from sheep vascular gland microsome | 6.25 μg/mL | [63] |
| Inhibition of LPS induced PGE2 release Inhibition of LPS induced IL-1 And NO (33% inhibition) release |
Macrophages J774A.1 | IC50: 11 μM | [64] | |
| IC50: 30.4 μM 32 μM | 69] | |||
| Inhibition of iNOS expression Blocking NF-κB/Rel pathway |
Macrophages Raw 264.7 | 3–10 μM | [69] | |
| Inhibition of LPS induced NO, TNF-α, IL-1β Blocking NF-κB and AP-1 DNA binding |
Murine microglia BV-2 cells | 0.3–10 μM | [65] | |
| Inhibition of calcymicin induced LTB4 and TXB2 production |
Promyelocytic leukemic HL-60 cells | IC50: 5.3 μM IC50: 11 μM |
[64] | |
| Inhibition of NF-κB and MAPK signaling, And TNF-α, IL-1β, IL-12, IFN-α/β release | Dendritic cells From BALB/c and C75BL/6J mice | 5–20 μM | [66] | |
| Attenuation of skeptic shock induce by LPS plasma level reduction of TNF-α and NO | BDF1 mice | 1–10 mg/kg | [68] | |
| Reduction of iNOS and COX-2 expression Suppression of MPO |
DSS induce colitis BALB/c mice colon tissue | 10 and 50 mg/kg | [67] | |
| Reduction of MCP-1 secretion | Crlj:CD(SD) rats | 10 mg/kg/2 days | [71] | |
| Anti-atherogenic | Inhibition of LDL oxidation (lipid peroxidation and oxysterols formation) | Plasma LDL E0 mice |
5–60 μM 50μg/day/mouse |
[17] [76] [39] |
| Reduction of atherosclerotic lesions | E0 mice | 20μg/day/mouse | [75] | |
| Reduction of cell mediated LDL oxidation Reduction of PKC, NADPH oxidase activities |
Macrophages J774A.1, MPM | 20 μM | [48] | |
| In vivo reduction of LDL oxidation | Human healthy volunteers | 60 mg/day | [78] [75] |
|
| Up-regulation of SOD, CAT and PON2 | Monocytes THP-1 | 0.1 μM | [80] | |
| Direct Interaction with PON1, Prevention of its oxidation | Recombinant PON1 | 10–100 μM | [81] | |
| Stimulation of DNA synthesis Proliferation of VSMC cells [estrogenic-like effects] |
Endothelial cells ECV304 Smooth muscle cells VSMC |
0.03–3 μM 0.003–0.3 μM |
[82] | |
| Inhibition of ICAM-1 and VCAM expression | Endothelial HUVEC | 3 mM | [83] | |
| Neuro-protection |
Inhibition of staurosporine induced DNA Fragmentation and apoptosis Up-regulation of Bcl-2 |
Primary cortical neurons | IC50:4.1 ±0.7 μM | [84] |
| Reduction of MDA production Increase of SOD and glutathione level |
Middle cerebral artery occlusion (MCAO) rats | 25 mg/kg during 7 days | [84] | |
| Increase of Acetylcholine level Decrease of choline esterase activity |
Kunming Mice | 2, 4 mg/kg during 3 days | [30] | |
| Improvement of learning and memory in non-Diabetic rats Reversion of deficits memory and diabetic rats |
Streptozotocine induced diabetic Wistar rats | 5, 25, 50 mg/kg during 30 days | [86] | |
| Inhibition of serotonin re-uptake (59.5 ± 5.8%) | HEK 293 cells | 50 μM | [88] | |
| Estrogenic-like (phytoSERM) |
Competitive ER binding No differentiation of ER subtype. |
T47D cells | IC50: ~5 μM | [91] |
| Biphasic effect on breast adenocarcinoma Inhibition of cell proliferation Growth stimulation (estrogenic like) |
MCF-7 cells | (E2 :0.1–10 nM) 15 μM 0.1–10 μM |
[91] | |
| Induction of wet uterus weight gain Induction of CK activity in Skeletal tissue(femur) Cardiovascular tissue (aorta, left ventricle) |
Wistar-derived Female rats | 200 μ g/rat during 24h 2.5–250 μg/rat during 24h |
[91] | |
|
No estrogenic response towards both ERs Subtypes. ERα-antagonist with 80% inhibition of the Estrogen response |
Yeats cells | 6 μM | [92] | |
| Induction of CK specific activity Stimulation of DNA synthesis Biphasic effect on cellular proliferation |
ECV 304 VSMC |
(E2 0.3–30 nM) 3 to 300 nM 30 to 3000 nM |
[82] | |
| Induction of CK specific activity in Cardiovascular tissue (aorta, left ventricle) | Wistar-derived Female rats | 2.5–250 μg/rat during 3 days, 2 weeks | [82] | |
| Induction of CK specific activity in pre- and Post-menopausal cells | Human osteoblasts | 3 μM (E2 30 nM) | [93] | |
| Induction of CK specific activity in Diaphyseal bone and epiphyseal cartilage | Prepubertal and ovariectomized female rats | 2.5–25μg/rat during 3 days, and 2 weeks | [93] | |
| Anti-osteoporotic | Promotion of cellular growth All ER-mediated induction of Phosphate alkaline activity, Synthesis of collagen, Secretion of osteocalcin | Murine Osteoblasts MC3T3-E1 |
1–10 μM | [96] |
| Stimulation of DNA synthesis Stimulation of CK specific activity Modulation of ERα/ERβ mRNA express. Increase of 1,25(OH)2D3 production |
Female-derived human bone cells hObs | 300 nM | [99] | |
| Inhibition of TNF-α induce NO and PGE-2 Releases and cellular apoptosis |
Murine Osteoblasts MC3T3-E1 Murine Osteoblasts MC3T3-E1 |
10 μM | [96] | |
| Reduction of Antimycin A induces cytotoxic effects by Increasing PI3K activity and Protecting mitochondrial integrity |
0.03–0.3 μM | [98] | ||
|
Reduction of dRib induces oxidative damages Restoration of PI3K and AKT2 expression Restoration expression of differentiation genes Up-regulation of SOD I and GPX 4 expression |
5 μM | [97] | ||
| Inhibition of c-SRc, PI3K, AKT2 expression Inhibition of RANKL induces MMP-9 and Cathepsin K expression Inhibition of osteoclast-associated genes |
RAW 264.7 differentiated in osteoclasts with RANKL | 2–5 μM | [102] | |
| Regulation of Energetic metabolism | Weight Reduction of white adipose tissue Regulation of genes involved in lipid Metabolism |
Fat-fed C75BL/6J mice | LFO 1–2% w/w (~1–2 ppm Glab) during 8 weeks | [106] |
| Abdominal fat lowering effect General hypoglycemic effect |
Obese diabetic KK-Aγ mice | LFO 2% w/w (~2 ppm Glab) during 4 weeks | [17] | |
| Stimulation of adipocyte differentiation | Human sub cutaneous adipocytes | LFO 1.2% w/w Glab 5–10 μg/mL | [17] | |
| Stimulation of AMPK through the regulation of mitochondrial activity: Decrease of adiposity Amelioration of lipid dysregulation Amelioration of insulin resistance |
Fat-fed C75BL/6J mice | 150 mg/kg/day during 4 weeks | [107] | |
| Genes regulation associated with Fatty acid oxidation Lipogenic genes Inflammation (inos and mcp-1) |
C2C12 myocytes FAO hepatoma cells 3T3-L1 adipocytes |
20–50 μM | [107] | |
| PPAR-γ binding activity | PPAR-γ | 2 and 10 μg/mL (~ 6 and 30 μM) | [12] | |
| Inhibition of adipogenic differentiation Up-regulation of PPAR-γ and C/EBPα Amelioration of hepatic steatosis Protection against fat diet induced weight gain and adiposity |
3T3-L1 C57BL/6J mice |
Glab : 25 μM LSC: 0.1, 0.25% w/w during 8 weeks |
[23] | |
| Increase of physical exercise duration Delay of the blood lactic acid increase Increase in glycogen storage in liver and muscle |
BALB/c mice | 5, 10, 20 mg/kg during 28 days | [108] | |
|
Increase in glucose tolerance and SOD activities Decrease in FBG level and MDA content in liver kidney, pancreas |
Streptozotocine induced diabetic Kunming mice | 10, 20, 40mg/kg during 28 days | [109] |
Bio. Systems: Biological systems represent the in vitro cellular system, the in vivo animal model, or the human study, chosen for the investigation
Active Conc.: Active concentration, or dose, is the concentration or amount of glabridin that lead to the significant measured bioactivity.
Bacterial lipopolysaccharide (LPS), a component of the gram negative bacterial membrane, is known to induce the overexpression of various inflammatory mediators in vitro and in vivo. In murine macrophages and microglia cells, Glab has been demonstrated to inhibit LPS induced PGE-2 [64], IL-1, NO [69], and TNF-α cellular release [65]. All these effects were shown to be directly linked to a reduction of COX-2 and iNOS expression. The latter was associated with a down regulation of major transcription factors, NF-κB [69], in both macrophages and microglia cells, where down-regulation of AP-1 was also implied [65]. In HL-60 promyelocytic leukemia cells, Glab also inhibited the production of potent inflammatory mediators, leukotriene LTB4 and thromboxane TXB2, in a concentration-dependent manner [64]. Similarly, anti-inflammatory effects observed in dendritic cells (DCs) were related to an inhibition of IκBα/β degradation, nuclear translocation of NF-κB p65/p50, and phosphorylation of elements from MAPKs signaling [66]. DCs are potent antigen-presenting cells that play a pivotal role in the innate and adaptive immune responses. Anti-inflammatory effects of Glab were also linked to a decrease in functional maturation of DCs, which according to Kim et al. [66] could have some interest for the regulation of the immune system especially in auto-immune diseases. A patent related to this research was issued in 2009 [70].
Kang et al. [68] suggested that Glab inhibits NF-κB/Rel activation through non-cell specific reduction of intracellular reactive oxygen species (ROS). Inhibitory effects of Glab on NF-κB activation has been found in different cell types and, therefore, seems to be universal. Park et al. [65] further corroborated this observation by demonstrating that the effect of Glab on AP-1 activity was differential in microglia and macrophages cells, suggesting that the modulation of AP-1 by Glab is cell-type specific.
Sepsis is a systemic response to infection triggered by an exposure to LPS. In vivo, Glab (1 to 10 mg/kg) was demonstrated to protect BDF1 mice against LPS-induced sepsis by reducing the production of various inflammatory mediators, such as TNF-α and NO [68]. Furthermore, oral administration of Glab (10 or 50 mg/kg during 7 days) to female BALB/C mice resulted in an attenuation of colonic inflammation induced by dextran sulfate sodium (DSS) [67]. In this colitis model, the activity of the enzyme MPO was shown to increase and found to be responsible for the formation of ROS leading to tissue damages. Glab treatment was associated with a suppression of MPO activity, thus contributing to the attenuation of inflammatory progression in the DSS-induced colitic colon. MCP-1 is known to contribute to macrophage infiltration in tissue, thus, maintaining their degradation. Non-obese/diabetic rats treated with Glab displayed a lower MCP-1 level compared to non-treated animals, indicating that Glab could also mitigate inflammation and reduce its deleterious effects on tissue through a reduction of MCP-1 secretion [71].
In conclusion, down regulation of NF-κB, cell specific down-regulation of AP-1, and MAPKS signaling, combined with a reduction of COX-2 expression, all participate in the cellular anti-inflammatory effects of Glab. On the other hand, suppression of MPO activity and MCP-1 secretion contribute to its protective effect against inflammation induced tissue damages. Altogether, these properties contribute to the various biological effects of Glab.
3.2 Prevention of LDL oxidation and anti-atherogenic properties
By far, the most studied biological property of Glab is its prevention of Low Density Lipoprotein (LDL) oxidation and its subsequent anti-atherogenic properties, which represent 23% of the current scientific literature dedicated to the bioactivities of Glab (Figure 1B). Atherosclerosis is characterized by the accumulation of cholesterol and oxidized lipid in the arterial wall. LDL is the major cholesterol carrier in human serum. Oxidized-LDLs are cytotoxic to the arterial wall through the stimulation of inflammatory and thrombotic processes and, therefore, are considered as highly atherogenic [72]. LDL can be oxidized by the major cell types present in the vascular wall, endothelial cells, macrophages, and smooth muscle cells. The possible cellular sources of LDL oxidants are, among others, NADPH oxidase and P450 enzymes [73]. Macrophage-mediated oxidation of LDL is considered to be of major importance during early atherogenesis, and the formation of oxidized LDL can then induce cellular cholesterol accumulation and foam cell formation (accumulation of ox-LDL in macrophages and other phagocytes, Figure 5) [74].
Figure 5. Effects of Glabridin on Various Biological Targets Involved in Atherogenesis.

The first established biological properties of Glab relate to its preventive effects on LDL peroxidation, which are involved in the development of atherosclerotic plaque in the arterial wall. Subsequently, it was demonstrated that anti-atherosclerotic properties of Glab result from its interactions with multiple targets involved in the maintenance of vascular integrity. Glab has also been shown to inhibit PKC, and NADPH oxidase, which are part of the LDL oxidation pathways, and is known to increase the activity of antioxidant enzymes such as SOD and CAT, as well as anti-atherogenic factors PON2 in macrophages and monocytes. Glab has also been demonstrated to interact with PON1, another anti-atherogenic factor, and protect him against peroxidation. Furthermore, Glab suppresses the cellular release of MCP-1 and inhibits the expression of adhesion markers ICAM-1 and VCAM, responsible for the infiltration of macrophages in tissue. However, most of these effects were observed for varying active concentration ranges of Glab. EC stands for Endothelium, OxLDL is oxidized LDL, ROS means Reactive Oxygen Species.
In a study by Vaya et al. [28], Glab (30 μM) has been demonstrated to possess antioxidant activity, compared to β-carotene consumption, against 2, 2′-azobis (2-amidino-propane) dihydrochloride (AAPH) induced LDL oxidation. Furhman et al. [75] demonstrated that Glab, when administered to atherosclerotic apolipoprotein E (E0) deficient mice for a period of 6–12 weeks (20 μg/day/mouse), was not only absorbed but also bound to the LDL particles. The compound reduced the formation of macrophage foam cells and prevented the development of atherosclerotic lesion areas. Moreover, the same authors demonstrated that Glab reduced the atherogenic modification of human LDLs (oxidation and aggregation) [75]. Furthermore, Belinky et al. [76] also analyzed the potential of Glab (50 μg/day/mouse) to inhibit the oxidation of LDLs obtained from E0 deficient mice. The authors confirmed that Glab effectively bound to LDLs and further concluded its capacity to inhibit the formation of lipids peroxides and oxysterol derivatives in LDL, regardless whether the oxidation was induced by copper ions or AAPH. According to preliminary structure activity relationship, the same authors suggested that antioxidant properties of Glab were mainly associated with its 2′,4′-dihydroxyl functions, whereas its hydrophobic prenyl moiety was assumed to favor its lipophilic interactions and inclusion in the LDL particles [39].
The protective effect of Glab against LDL oxidation was further investigated by Rosenblat et al. [48], who suggested that the 50% reduction of the aortas atherosclerotic lesions observed in treated E0 mice was, in fact, related to the effect of Glab on macrophages mediated LDL oxidation. Therefore, the authors also analyzed the macrophage ability to take up Glab and studied the mechanisms by which cellular accumulation of Glab can affect macrophage-mediated oxidation of LDLs [48]. Interestingly, the results indicated that Glab accumulated in macrophages both in vitro and in vivo, and reduced the cell-mediated oxidation of LDLs at almost ~ 90%. This effect was linked to an intracellular inhibition of protein kinase C (PKC) activity (70% inhibition), which had a subsequent inhibitory effect on the translocation of the NADPH oxidase P-47 cytosolic component to the plasma membrane and, hence, on macrophage superoxide production. Additionally, Kent et al. [77] reported the inhibition of cytochrome P450 3A4 by Glab and concluded that this effect may contribute to the protective effect of Glab against LDL oxidation and its subsequent antiatherosclerotic properties (see section 4.1). The in vivo inhibitory effect of Glab on human LDL oxidation was investigated by Carmelli et al. [78]. In this study, administration of Glab (60 mg) through a standardized crude extract from G. glabra (Licolife™) to 22 healthy volunteers and during 6 months resulted in a 20% reduction of plasma LDL oxidation.
Paraoxonase 2 (PON2) is expressed in many different types of tissues and cells, including cells of the arterial wall, e.g., macrophages, and demonstrates antioxidant properties [79]. Glab (0.1 μM) was shown to have the potential to up-regulate antioxidant enzymes including manganese sodium oxide dismutase (SOD), catalase (CAT) and PON2 under glucose stress in human monocytes (THP-1), which are precursors of macrophages [80]. Together with glutathione peroxidase (GPX), SOD and CAT are the major vascular antioxidant defense enzymes. The authors suggested that Glab could, therefore, strengthen the antioxidant defense mechanism and may serve as an anti-atherogenic agent. Paroxonase 1 (PON1) is another anti-atherogenic enzyme forming part of the circulating HDL (High Density Lipoprotein). Its activity is inhibited through an oxidation process initiated by the lipid fraction of the human carotide plaque or by linoleic acid hydroperoxide [79]. Hatrahimovich et al. [81] demonstrated that Glab specifically interacted with the enzyme and consequently reduced or prevented its inhibition by linoleic acid hydroperoxide.
In relation with its estrogenic-like properties (see section 3.4 and figure 6), Glab has been demonstrated to stimulate the proliferation of vascular smooth muscle and endothelial cells, two fundamental mechanisms for the healing of damaged vascular endothelium [82].
Figure 6. Comparative Structures of Estradiol (E2) and Glabridin and Effects on Bone and Vascular Tissues.

According to Somjen et al. [82], several features are common to the structures of Glab and 17β-estradiol (E2). Both have an aromatic ring substituted with a hydroxyl group (C-4′ for Glab or C-3 for E2), and share three fused rings with an overall phenanthrene-like shape. Both molecules are also relatively lipophilic, containing additional oxygen, although in adjacent positions (17 in E2, 1″ in Glab). These characteristics features could explain the binding of Glab to ERs leading to health beneficial effects in estrogen-responsive tissues such as bone and vascular tissues. Glab has been demonstrated to exert ER mediated osteogenic effects by stimulating the functions of osteoblasts (Oblast), while inhibiting the development of osteoclasts (Oclast). In addition to its anti-atherogenic properties, Glab has been shown to stimulate the proliferation of vascular smooth muscle cells (SMCs) and endothelial cells (Endo), altogether promoting the vascular plasticity.
Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are protein adhesion markers which play pivotal roles in the development of atherosclerosis and in the plaque destabilization. These proteins are known to recruit the circulatory inflammation cells and facilitate their migration into the arterial walls [5]. When cultured human umbilical vein endothelial cells (HUVEC) were exposed to TNF-α, Glab (3 mM) produced a 50% inhibition of the endothelial cell expression of ICAM-1 and VCAM-1 [83].
In summary, the antiatherosclerotic properties of Glab result from its beneficial effects on various targets involved in the maintenance of vascular integrity. This literature survey presents ample evidences that Glab is capable of down-regulating intracellular pro-oxidant proteins (PKC, NADPH oxidase), up-regulating antioxidant defense systems (SOD, CAT, PON2), and interacting with anti-atherogenic PON1, thus inhibiting cellular mediated LDL peroxidation. Moreover, Glab has been shown to inhibit the expression of protein markers ICAM-1 and VCAM-1 at elevated concentrations, while generally increasing the proliferation of vascular endothelial cells and, therefore, reducing vascular inflammation and favoring healing process of atherosclerotic lesion (Figure 5).
3.3 Neuroprotective effects
Oxidative stress, inflammation and subsequent neuronal apoptosis (programmed cell death) can occur after a brain ischemic insult. Excessive production of inflammatory mediators in brain causes neurotoxicity in various neurodegenerative diseases such as Alzheimer’s, and Parkinson’s diseases or senile dementia. Neuroprotective effects of Glab were demonstrated to be associated mainly to its anti-inflammatory functions [65] and its capacity to down-regulate intercellular oxidative stress by increasing the expression of antioxidant makers [84]. Hence, Glab was shown to protect cellular (neuronal) functions against damages (DNA fragmentations, protein oxidations, mitochondrial peroxidation) that lead to apoptosis [85].
On cortical neurons, Glab was shown to inhibit staurosporine induce DNA fragmentation and apoptosis [84]. Its protective effects were further confirmed in vivo on a reversible model of cerebral ischemia (i.e, Middle Cerebral Artery Occlusion [MCAO] rat models), where Glab treated rats displayed a reduced total infarct volume associated with a reduction of malondialdehyde (MDA, maker of lipid peroxidation) levels and an increase in antioxidant markers such as SOD and glutathione [84]. Altogether, these effects were associated with inhibition of oxidative stress induced neuronal damages.
Investigations carried out by Cui et al. [30] demonstrated that mice treated with Glab (2 and 4 mg/kg) during 3 days presented a general improvement of their memory. Interestingly, these positive effects were associated with an increase in brain acetylcholine levels and a decrease in choline esterase activity. Similarly, Glab was also demonstrated to reverse learning deficits of streptozotocine-induced diabetic rats, without altering their hyperglycemic status [86]. It was, therefore, assumed that Glab might exert a beneficial effect on various neurodegenerative diseases, including cognitive impairment linked to mellitus diabetes and Alzheimer’s disease, not only through the protection of neuronal cells from oxidative stress and inflammatory damages, but also via potential anticholinesterase properties. A composition for the treatment of mental health disorders containing Glab has been patented in 2006 [87].
Additionally, Glab was found to be an effective inhibitor of serotonin re-uptake in HEK 293 cells [88]. An approximate 60% inhibition of serotonin re-uptake was observed at 50 μM, compared to a 74% inhibition for imipramine at 5 μM. Selective blockage of the serotonin receptors in the human central nervous system (CNS) is considered to be the initial step in the pharmacological improvement of a wide variety of disorders, including major depression [89]. The authors suggested that Glab could have a beneficial effect on the mild to moderate depression, especially among pre-post-menopausal women.
Nevertheless, despite interesting neuroprotective activities, the bioavailability of Glab in the brain could be compromised due to its interaction with P-glycoprotein (P-gp), which is expressed notably in capillary endothelial cells. As demonstrated by Yu et al., this protein limits Glab penetration through the blood-brain barrier and consequently reduces its bioavailability in the CNS [90]. The interaction of Glab with P-gp would translate into a higher dosage required to reach efficacious levels of Glab for the treatment of CNS diseases, and might also be responsible for Glab-drug interactions (see section 4.2).
3.4 Estrogenic properties: is glabridin a potential PhytoSERM?
Diverse estrogen-like activities have been reported for Glab, which has been compared in structure and lipophilicity with 17β-estradiol (Figure 6). Glab binding to the estrogen receptors (ERs) has been evaluated in vitro on human breast cancer cells [91] and in yeast-based assays [92]. Its estrogenic-like properties have also been evaluated, in vitro and in vivo, on skeletal and cardiovascular tissues of female rats [82, 93] by measuring the induction of the specific activity of creatinine kinase (CK), an estrogen-induced enzyme. The present literature survey clearly indicates that Glab shows assay-dependent estrogenicity (Table 3).
Tamir et al. [91] have investigated the capacity of Glab to bind to ERs and measured its effects on estrogen responsive human breast cancer cells (MCF-7, MDA-MB-231 and T-47D). The authors found that Glab was a weak ligand to the ER (IC50 ~5 μM without differentiation of ER sub-type) and has a biphasic effect on the proliferation of breast cancer cells (MCF-7 and T47D cells). In vivo observations indicate that Glab induces the estrogen response marker CK in rat skeletal, cardiovascular tissues, and promotes uterus wet weight gain. The authors concluded that Glab represents a phytoestrogen which acts as estrogen agonist in estrogen-responsive tissues such as bone and vascular tissues (Figure 6).
Completing these picture of observations on the estrogenic-like properties of Glab, Somjen et al. further analyzed the capacity of Glab to induce CK in the same vascular, and skeletal tissues [82, 93]. In both studies, Glab showed effects similar to 17β-estradiol (E2), although at higher concentration, by inducing the estrogen response marker CK in vitro and in vivo. Glab stimulated DNA synthesis in endothelial cells ECV-304 (30 to 3000 nM), and displayed a bi-phasic effect on the proliferation of VSMC smooth muscle cells (3 to 300 nM). Following all these results on the estrogen-like activities of Glab and its derivatives, Vaya filed a patent on the phytoestrogenic pharmaceutical preparations comprising flavonoids from licorice, particularly Glab and its derivatives [94].
Using a yeast estrogen bioassay, in which observed responses are specific for effects directly mediated by the ERs, Simons et al. tested the estrogenic-like activities of Glab [92]. As a result, Glab did not initiate any significant estrogenic response on both ER subtypes. However, Glab was demonstrated to be a selective ERα antagonist with 80% inhibition of the estrogen response at 6 μM. Considering the agonistic activity of Glab in the MCF-7 cell proliferation assay [91], in two in vivo animal models [82, 93], and its ERα-selective antagonistic activity in the yeast estrogen bio-assays, the authors concluded that Glab might act as a SERM regarding its dual mode of action [92, 95]. These tentative conclusions regarding the potential phytoSERM properties of Glab were shared by Somjen et al. [93], who corroborated previous finding by studies demonstrating that Glab has potent osteogenic activities.
3.5 Anti-osteoporotic properties
Estrogen deficiency is known to be involved in osteoporosis. According to Somjen [93], Glab can act as a phytoestrogen and yield the same beneficial effects on bone tissues as E2 or genistein, an isoflavone. Compared to genistein, Glab is effective at lower concentration differing by a factor 10, which emphasizes the potential of isoflavan derivatives to modulate bone disorders in post-menopausal women.
The osteogenic activities of Glab has been analyzed in vitro on murine osteoblast cells MC3T3-E1 [96–98], but also on human female derived bone cells (hObs) [99]. In these studies, Glab was demonstrated to increase the function of osteoblasts, which are key cells in bone matrix formation and calcification. Beneficial effects were demonstrated to be ER mediated [96, 99] and associated with cellular protection against oxidative burst, through an up-regulation of antioxidant enzymes (SOD I and GPX 4) [97], thus, preventing the alteration of mitochondrial functions [100, 98].
Optimum bone growth and prevention of osteoporosis in postmenopausal women require adequate concentrations not only of estrogens but also of vitamin D3 (1, 25(OH)2D3). When testing on human female osteoblasts at 300 nM, Glab and its derivative, glabrene, have been demonstrated to favor the production of 1,25(OH)2D3 which in turn up-regulated cellular sensitivity to E2 [99]. Moreover, Glab has been shown to restore the expression of osteoblastic differentiation genes, and increase the expression of phosphatidylinositol-3′kinase (PI3K) and protein kinase B 2 (AKT2) genes [98, 97], which play a critical role in cellular plasticity, growth, and survival [101]. Anti-inflammatory properties of Glab, tested on murine osteoblasts, also contribute to its beneficial effects through the inhibition of PGE2 and NO production and the prevention of osteoblasts apoptosis induced by TNF-α [96].
Acting in a complementary manner to its effects on osteoblasts, Glab has been demonstrated to inhibit RANKL induced osteoclastogenesis in murine macrophages, RAW 264.7 [102]. In these differentiated bone resorbing cells, Glab was shown to down-regulate the expression of osteoclast related genes and reduce the expression of PI3K, AKT2. These negative effects on osteoclast were proposed to be related to an inhibition of the transcription factors AP-1/c-Fos via blockage of MAPKs signaling.
In summary, Glab exerts antioxidant activities in osteoblasts and improves their differentiation function, whereas in osteoclast it reduces the expression of genes related to their bone resorbing properties. Proven ER-dependent osteogenic effects and its potential to reduce inflammation suggest that Glab might play a positive role in the prevention of osteoporosis induced by estrogen deficiency, and in the regulation of localized bone destruction associated with inflammatory bone diseases.
3.6 Regulation of Energy Expenditure and Metabolism
Disturbances in energy homoeostasis underlie a number of disease states in humans such as type-2 diabetes and obesity, which can lead to hypertension and cancer. Glab has been proven to participate into the regulation of energy expenditure through mainly two types of interactions, the stimulation of AMPK activity and the up-regulation of PPAR-γ. Adenosine monophosphate activated protein kinase (AMPK) is a master energy sensor, which regulates diverse metabolic pathways, increases mitochondrial activity and biogenesis in muscle, and is responsible for the inhibition of adipogenesis [103, 104]. PPAR-γ is a ligand-dependent transcriptional regulatory factor, known to regulate the expression of a group of genes that maintain glucose and lipid metabolism. PPAR-γ also participates into the programmed differentiation of adipocytes [105]. Altogether, the stimulation of AMPK and up-regulation of PPAR-γ lead to the regulation of genes implied in lipid metabolism and adipocytes differentiation, eventually decreasing adiposity and reducing insulin resistance (Table 3).
The earliest reports on the regulation of energy metabolism by Glab were focused on the biological evaluation of LFO extract standardized to ~1% w/w of Glab [1, 17, 106]. This extract has been tested on two mice models: fat-fed induced mice (C57BL/6J mice) and obese diabetic KK-Ay mice. LFO extract has been reported to suppress the expression of genes involved in lipogenesis [106], leading to a decrease in both abdominal-fat and blood sugars [17]. However, no unambiguous evidence could be obtained that Glab might be associated or mainly responsible for these beneficial effects.
Lee et al. have studied the direct effect of Glab on AMPK activity in obese rodents models (C57BL/6J mice)[107]. They observed that Glab decreases adiposity and ameliorates lipid dysregulation as well as insulin resistance, thus, lowering body weight. The molecular mechanism underlying these effects were demonstrated to be associated with an attenuation of mitochondrial ATP production, leading to an increase in the cellular AMP contents, hence, promoting AMPK activation. In muscles, Glab was shown to regulate genes associated with fatty acid oxidation, and to reduce the expression of lipogenic genes in liver and adipose tissues, where it also decreased the expression of pro-inflammatory genes (inos, mcp-1).
A total of 39 licorice constituents have been tested for their PPAR-γ ligand binding activity. Among them, 14 phenolics including Glab and shinpterocarpin displayed significant PPAR-γ binding, suggesting that these compounds, and the hydrophobic crude extract containing them, can potentially have anti-diabetes and anti-obesity properties mediated by the activation of PPAR-γ receptors [12]. Recently, Ahn et al. confirmed this hypothesis by demonstrating that Glab (25 μM) inhibited the adipogenic differentiation of mice fibroblast 3T3-L1. However, there is no evidence that Glab alone can effectively up-regulate PPAR-γ in this cellular model. A supercritical CO2 extract of G. glabra (LSC) enriched in Glab (4.5% w/w Glab) was shown to inhibit adipogenic differentiation of fibroblasts through an up-regulation of PPAR-γ and C/EBPα. In vivo, LSC (0.1 and 0.25% w/w during 8 weeks) was responsible for a dose-dependent reduction of weight gain of treated C57BL/6J mice, and was demonstrated to regulate genes involved in hepatic metabolism [23].
In complement of these effects on cellular regulation of energy metabolism, Glab has also been shown to be effective in the reduction of mice physical fatigue associated with exercise by delaying lactic acid production and increasing glycogen storage in muscle and liver [108]. Additionally, direct hypoglycemic effects of Glab were investigated in vivo using an animal model of diabetes mellitus [109]. Data revealed that mice treated with Glab over a period of 28 days displayed a better glucose tolerance associated with a surprising increase in their body weight, compared to other studies. Moreover, the sodium oxide dismutase activity was increased, while the level of fast blood glucose (FBG) and MDA were decreased in the liver, kidney, and pancreas. Taken altogether, these results indicate clearly that Glab itself possesses hypoglycemic effects. However, the mechanism of action underlying these properties remains to be investigated. Finally, licorice flavonoids have been tested for their capacity to inhibit the pancreatic lipase and reduce intestinal lipid absorption. Glab did not show any significant inhibition (IC50: 485.6 μM) of this enzymatic activity [110].
In summary, there is strong in vitro and in vivo evidence that Glab and licorice extracts enriched in this compound can stimulate AMPK and participate to the up-regulation of PPAR-γ. Both effects contribute to the reduction of adipogenesis and favor insulin sensitivity. In vivo, Glab has been demonstrated to enhance glucose tolerance of mice as well as glycogen storage in liver and muscle. Consequently, both pure Glab and Glab-enriched extracts were found to have therapeutic potency for obesity-related metabolic diseases, which is further supported by the observation that Glab itself exhibits anti-atherogenic and anti-inflammatory activities. The latter are known to be linked to numerous secondary clinical effects of obesity. Considering the importance of metabolic related diseases in developed countries and their impact on the DS market, several extracts of licorice enriched in Glab have been patented for the treatment of metabolic disorders [111][112][113].
3.7 Less frequently reported biological activities
3.7.1 Anti-cancer and chemo-preventive properties
Anti-cancer properties of Glab can be classified into two different mechanisms: inhibition of the integrin/FAK/Src pathway, which plays an important role in tumor metastasis [114, 115]; and inhibition of P-glycoprotein (P-gp) [116], which acts as an anticancer drug efflux transporter in cancer cells [117] (Table 4).
Table 4.
Less Frequently Reported Bioactivities of Glabridin
| Reported Activities-Targets | Bio. Systems1 | Active2 Conc. | Ref. | |
|---|---|---|---|---|
| Chemo-prev |
Reduction of intracellular ROS and DNA Fragmentation induced by UVB Up-regulation of Bcl-2 Inhibition of p53 |
Normal Human Keratinocytes NHK | 15 μM | [118] |
| Anti-cancer | Inhibition of cellular migration and adhesion Inhibition of tumor cells angiogenesis (invasion of HUVEC, blood endothelial cells) Through inhibition of Fak/src pathway |
human non-small cells lung cancer A 549 |
1–10 μM | [114] |
| Breast Adenocarcinoma cells MDA-MB-23 |
1–10 μM | [115] | ||
| Inhibition of P-gp, increasing Daunorubicin Concentration. | KB-C2, drug resistant cells | 50 μM | [116] | |
| Anti-tyrosinase | Inhibition of tyrosinase isozymes T1 and T3 | B16 murine melanoma cells | 0.1 to 1 g/mL | [63] |
| Reduction of UVB induce erythema and Pigmentation | Guinea Pig skin | formulation at 0.5% w/w | [63] | |
| Inhibition of tyrosinase | Tyrosinase enzyme | IC50: 2.93 μM | [121] |
Bio. Systems: Biological systems represent the in vitro cellular system, the in vivo animal model, or the human study, chosen for the investigation
Active Conc.: Active concentration, or dose, is the concentration or amount of glabridin that lead to the significant measured bioactivity.
Related to the first mechanism, Glab has been shown to inhibit the migration and adhesion of human non-small cell lung cancer cells, A549, and human breast adenocarcinoma cells, MDA-MB-23, in a concentration dependent manner. Glab also decreased the invasion of blood endothelial cells HUVEC (Human Umbilical Vein Endothelial) through the concomitant inhibition of the integrin/FAK/Src pathway, and effectively inhibited in vivo tumor cell angiogenesis [114, 115]. According to the second mechanism, Glab was reported to enhance the efficacy of cancer chemotherapy by inhibiting P-gp (50 μM), but not MDR-1, another drug efflux transporter, on KB-C2 cells (human multidrug resistant carcinoma), therefore, increasing the intracellular concentration of daunorubicin [116]. Associated with its phytoestrogenic properties [91], Glab has also been demonstrated to decrease the growth of human breast cancer cells (MCF-7, MDA-MB-231 and T-47D) at concentrations above 15 μM (Table 3, section 3.4).
In addition to these effects, Glab displays chemo-preventive properties not only through its ability to reduce the intracellular oxidative burst and UVB induced DNA damages on normal cells (neurons, osteoblasts and keratinocytes) [84, 98, 118], but also through its general anti-inflammatory properties and its capacity to inhibit the expression of COX-2 enzyme (see section 3.1).
3.7.2 Antinephritic effects
Fukai et al. have evaluated the effects of a Glab treatment during 10 days (30 mg/kg/day) on BALB/c mice with glomerular disease. The authors observed a reduction of the total protein excretion and the serum cholesterol level for the treated mice. Electron Spin Resonance (ESR) spectroscopy demonstrated that Glab neither produced radicals, nor affected the radical intensity of sodium ascorbate. This led to the conclusion that the antinephritic effect of Glab was not correlated with its known radical scavenging activity [119]. The molecular mechanism explaining this biological activity remains to be elucidated.
3.7.3 Inhibition of melanogenesis
Tyrosinase acts as a rate-limiting enzyme on the sequential pathway to melanin formation. Therefore, tyrosinase inhibitors may be of importance to treat abnormal pigmentation disorders and act as skin whitening agents in cosmetics [120]. At a relatively high concentration range of 0.1 and 1 g/mL, Glab has been demonstrated to inhibit the activity of tyrosinase isozymes T1 and T3 implied in melanogenesis in B16 murine melanoma cells [63]. Nevertheless, in an enzymatic in vitro assay [121], Glab displayed a better tyrosinase inhibition (IC50:2.3 μM) than the widely used kojic acid (IC50:43.7 μM). Topical application of 0.5% Glab to the skin of guinea pigs was shown to reduce UVB induced erythema and pigmentation [63] (Table 4). Interestingly, the authors also indicated that hydroxyl functions in position C-2′ and C-4′ were necessary for the anti-tyrosinase effects of Glab.
Some articles [56, 57] and numerous patents are dedicated to the application and formulation of Glab for cosmetic or dermatologic outcomes [122, 123]. In some of the cosmetic preparations, Glab has been chemically modified to favor its skin bioavailability and ease the formulation process [50, 51]. For that purpose, various structural derivatives, with the 2′- and 4′-hydroxyl functions protected by ester or ether derivatives, were synthetized as tyrosinase inhibitors [40] (see section 2.5 and 2.6). Interestingly, cosmetic patents represent 70% of all patents dedicated to Glab applications (Figure 1C).
3.7.4 Antibacterial and antifungal activities
Spectrum antibiotic activities of Glab have been described in relatively few articles and patents. Reported minimum inhibitory concentrations (MICs) range from 3.13 to 25 μg/mL (except for the resistant mutants of C. albicans). Considering the MIC ranges and spectrum of microorganisms tested, it can be concluded that Glab unfold only weak and non-specific antibiotic properties (Table 5) [31, 124–129]. Testing against C. albicans, Glab was demonstrated to inhibit the yeast-hyphal formation and to act in synergy with nystatin [126]. Except for this particular activity, its antibiotic mechanism of action has neither been explained nor been investigated, likely due to its weak potential.
Table 5.
Spectrum Antibiotic Activities Reported for Glabridin
| Microorganism | Strains | 1 MIC in μg/mL (μM) | Ref. |
|---|---|---|---|
| Staphyloccocus aureus | ATCC 13709 FDA 209P and Smith |
6.25 (20) 3.13 12.5 (10–40) |
[31] [124] |
| Methycyllin Resistant S. aureus | Strains K3 and ST 28 | 3.13–12.5 (10–40) | [124][125] |
| Mycobacterium smegmatis | ATCC 607 | 6.25 (20) | [31] |
| Candida albicans | ATCC 10231 | 25 (80) | [31] |
| ATCC 28366 and LAM-1 | 12.5 (40) | [126] | |
| Drug Resistant mutants (of amphotericin, clotrimazole and nystatin) | 31.25–250 (100–800) | [127] | |
| Helicobacter pilory | ATCC 700392 | 12.5 (40) | [128] |
| Corynospora cassiicola | Not described | 12.5 (40) | [129] |
MIC: Minimum Inhibitory Concentration.
Asha et al. have evaluated Glab and a flavonoid enriched extract of G. glabra (GutGard®, containing ≥ 3.5% w/w of Glab) against different strains of Helicobacter pylori, which is considered as the leading cause of peptic ulcer disease [128]. MICs measured for Glab and GutGard® were 12.5 and 100 μg/mL, respectively. Inhibition of protein synthesis, DNA gyrase and dihydrofolate reductase could possibly explain the anti-H. pylori mechanism of action of GutGard® extract. The mechanism of action was described for GutGard®, but not specifically for Glab.
In association with its phytoalexin properties, an oily extract of licorice, which contains Glab as active component, has been patented as a fertilizer and plant disease-preventing agent. It was demonstrated that Glab at 12.5 μg/mL gave 100% inhibition of spore germination from the tomato pathogen Corynospora cassiicola [129].
4 Pharmacokinetic parameters
4.1 Metabolism and Cytochromes P450
Kent et al. have investigated the effect of G. glabra extract and Glab, on the enzymatic activities of several human P450 enzymes such as P450 3A4, 2B6 and 2C9, which play a key role in the metabolism of clinically used drugs [77]. The isoform P450 3A4, which represents the major hepatic and intestinal P450 enzyme in humans, is known to metabolize more than 50% of clinically used drugs. In the cited study, cytochromes were expressed and purified from E. coli, then reconstituted for the enzymatic assays which were carried out with different concentrations of Glab. Interestingly, the reconstituted cytochromes 3A4 and 2B6 were inhibited by Glab in a time-, concentration- and NADPH-dependent manner, and the concentration required to obtain 50% inhibition was determined to be 7 and 12 μM, respectively. Both inhibitions were found to be irreversible through dialysis, and for the isoform 3A4 the inhibition was associated with the destruction of the heme moiety. Additionally, the authors suggest that metabolism of Glab by the cytochrome 2B6 could generate reactive intermediates that might bind to the apoprotein. Nevertheless, the identity of the reactive intermediates produced by the cytochromes has not been defined. As 2′,4′-dimethylgabridin does not interact with the isoform 3A4, Kent et al. suggested that the two hydroxyl groups on the 2′ and 4′ position of the B ring, which are believed to be essential for most of Glab biological activities [39, 130], are also required for P450 3A4 inactivation. Further investigations will be necessary to define the interactions of Glab with other P450 isoforms and identify its metabolites.
4.2 Metabolism and Intestinal Absorption
A study by Cao et al. [131] reported that Glab is a substrate of the intestinal p-glycoprotein, P-gp/MDR1. Glab metabolism in rat intestinal and hepatic microsomes was also investigated and revealed that Glab was mainly metabolized by glucuronidation, and that the metabolic capacity of intestinal microsomes was 1/15 to 1/20 of that of liver microsomes. Systemic bioavailability of Glab after oral administration at 5 and 20 mg/kg was found to be very low, only about 7.5% of the Glab was absorbed. The same authors concluded that low water solubility, intestinal transporter-mediated efflux via P-gp, and first-pass metabolism through glucuronidation could be considered as factors responsible for the overall low oral bioavailability of Glab.
Different results were presented by Ito et al. [71], who evaluated the absorption of Glab by a human colon adenocarcinoma cell line (Caco-2). The authors demonstrated that intact Glab was released to the basolateral side mostly without metabolic conversion and with a 90% recovery rate when administered alone, or at a rate of 82% when administered in the form of LFO enriched extract. In rats, dietary Glab (oral dose of 10 mg (30 μmol)/ kg) was partly incorporated into the blood and liver in an unchanged form. Glab showed a maximum concentration 1 h after the dose administration at 87 nmol/L for standard non-conjugated Glab, and decreased gradually over 24 h after dosing. In vivo, the intestinal absorption of Glab was higher when administered in the form of LFO compared to the pure compound. Moreover, Glab seemed to escape conjugation during intestinal absorption and appeared in the blood stream as the unaltered aglycone. Based on these results, the authors concluded that Glab possesses high oral bioavailability.
These two apparently conflicting studies indicate that further investigations on Glab intestinal absorption and metabolism are warranted for a better understanding of its bioavailability. Interestingly, absorption or PK studies of Glab following administration of powdered roots or other commercial extracts from G. glabra have not been reported to date. This type of study would be very interesting to consider, given that commercial DSs mostly contain either powdered roots or their crude extracts rather than a single chemical entity.
4.3 Pharmacokinetics in healthy humans
Aoki et al. have also studied some pharmacokinetic parameters in healthy humans when administering Glab in the form of LFO (1% w/w of Glab) [132]. On the basis of the hematological (e.g., hematocrit, platelet, red blood cells) and relevant biochemical (e.g., total protein, albumin, bilirubin, creatinine) results obtained from healthy volunteers, the authors concluded that a dose of LFO up to 1200 mg/day appeared to be safe and well tolerated. Additionally, their results indicated that Glab exhibited linear pharmacokinetics over the dose range of 300 to 1200 mg/individual of LFO. The steady state plasma level of Glab (1.42–4.4 ng/mL) was reached after two weeks of daily administration. Regarding these plasma concentrations, Aoki suggested that inactivation of the major cytochrome P450s by Glab should be minimal when daily doses of at least 300 to 1200 mg of LFO would be administered for 4 weeks. However, the same team also recognized that other flavonoids of the extract may additively or synergistically inactivate cytochromes P450, leading to an overall complex situation.
Despite several documented studies, the PK parameters of Glab and determination of its metabolism are only in their beginnings, and further studies will be necessary to better define its bioavailability, existence of potential bioactive metabolites, and precise profile of its P450 interactions.
5 Conclusions
Several methods for the extraction of G. glabra roots and enrichment in Glab have been developed. The most widely used extraction resort to the use of EtOH and supercritical CO2. Reported techniques for the isolation of this phytoconstituent remain complex. They all require approximately four mechanistically different steps, are time and solvent consuming, relatively difficult to scale-up, and do not provide a predictable outcome guarantee for acceptable yield and purity. To date, there is no truly targeted, rapid and reproducible method for the efficient isolation of this chemical and biological marker. While synthetic methods may produce large amount of Glab, documented procedures typically produce enantiomeric mixtures which require a follow-up by chiral separation to yield enantiopure Glab.
The comprehensive spectral characteristics (NMR, UV, CD, X-ray) of Glab gathered in this review provide useful information for the rapid structural dereplication of this compound. Moreover, specific analytical HPLC (UV and MS detections) and HPTLC methods have been proposed for the quantitation of Glab in herbal products and blood samples. However, it is important to recognize that Glab is known to be chemically unstable. In order to enhance its stability and ease its formulation, several glabridin 2′ and 4′ ester or ether derivatives have been synthesized. One relatively unexplored area in the phytochemical characterization of Glab is the identification of its degradation products. These compounds may be present in the crude extract of G. glabra. A second area for potential improvement is the development of a reproducible and scalable method for the targeted and efficient purification of Glab. Additionally, the purity determination of isolated or synthetized Glab has not always been consistently addressed. Both identity and purity of the investigated compound should always be considered in biological studies for meaningful interpretation of the final results [133].
All Glycyrrhiza species considered, Glab is by far the most studied flavonoid of licorice botanicals. Since 2005, most articles related to the title compound were on the evaluation of its bioactivities. Its anti-atherogenic properties have been exhaustively described as being linked to its antioxidant properties towards biological membranes, and its capacity to favor the healing process of lesion areas. The latter effect is partly regarded as a consequence of its phytoestrogenic properties, which are also associated with its preventing effects on osteoporosis. To this end, Glab is considered as a potential phytoSERM. Nevertheless, more experiments are warranted on different estrogen-responsive cell types to confirm this potential effect. The capacity of Glab to inhibit the production of pro-inflammatory factors, up-regulate antioxidant enzymes, and protect mitochondrial functions against oxidation, all contribute to the protection of cellular integrity and are part of its array of bioactivities related to anti-atherogenesis but also neuroprotection and anti-osteoporosis (Figure 7A). The regulation of intracellular oxidative burst also participates in the general chemo-preventive effect, which merits additional investigations. Furthermore, Glab has been demonstrated to participate into the regulation of cellular energetic metabolism through an activation of AMPK and potentially up-regulation of the nuclear factor PPARγ (Figure 7B). In this regard, the direct effect of Glab on the in vitro/vivo stimulation of PPAR-γ also deserves further investigation. According to the present literature survey, antinephritic, antibacterial, and anti-cancer properties of Glab can be regarded as less investigated activities and appear to result from other, yet to-be-determined molecular mechanisms.
Figure 7. Principal Biological Activities Reported for Glabridin and Beneficial Effects on Metabolism Syndrome (Syndrome X).
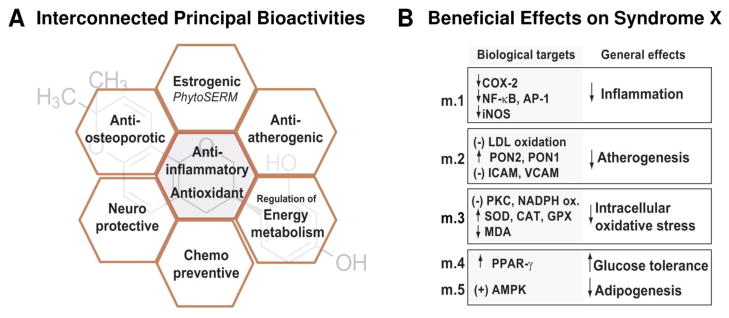
A) The capacity to reduce intracellular oxidative stress combined with anti-inflammatory properties produce the common denominator of all major bioactivities of Glab. Therefore, these two bioactivities constitute the core of the figure. Interaction of Glab with ERs contributes to its beneficial osteogenic (anti-osteoporotic) and anti-atherogenic effects. Glab also protects normal cells such as neurons or osteoblasts against inflammation, oxidative stress and apoptosis, thus, highlighting its chemo-preventive activities. The neuroprotective property of Glab is not directly related to its anti-osteoporotic effects, as represented by the gap between the two hexagons. Similarly, chemo-preventive activities of Glab are not directly linked to its capacity to regulate the energy metabolism. Through its activation of AMPK and potentially PPAR-γ, Glab can regulate the energy metabolism, thus, reducing adipogenesis and increasing insulin sensitivity. This property, together with the prevention of atherogenesis, also participates in its positive influence on the metabolism syndrome. B) According to Jungbauer et al. [62] phytoestrogens, such as Glab, counteract the cellular derailments that are responsible for the development of metabolic syndrome (syndrome X), mainly through five different mechanisms. Each of them is linked to the modulation of different biological targets leading to the reduction of inflammation (m.1), atherogenesis (m.2), and oxidative stress (m.3), while favoring glucose tolerance, insulin sensitivity (m.4), and reducing adipogenesis (m.5).
6 Discussion
All major biological activities attributed to Glab (anti-atherogenic, anti-inflammatory, estrogenic, anti-osteoporotic, regulation of energetic metabolism) seem to be interdependent and affect targets far beyond estrogen-related diseases (Figure 7A and B). A recent review emphasized that the multiple biological properties of phytoestrogens mainly counteract the cellular derailments that are responsible for the development of metabolic syndrome [62]. Also called syndrome X, this syndrome is a combination of disorders that, when occurring together, increase the risk of diabetes and cardiovascular diseases development [134]. According to the review by Jungbauer and Medjakovic [62], the effects of phytoestrogens on metabolic syndrome could be explained by five molecular or cellular mechanisms (m.1-m.5), which are mostly associated with the activation of nuclear receptors PPARs:
-
m.1
Down-regulation of the pro-inflammatory cytokines, COX-2, and iNOS
-
m.2
Increase of reverse cholesterol transport (mediated through PPAR-γ)
-
m.3
Activation of antioxidant genes (through KEAP)
-
m.4
Increase in insulin sensitivity (through PPAR-α by up-regulation of GLUT-4)
-
m.5
Increase in energy expenditure (signaling cascade of AMPK)
It is interesting to note that the reported biological activities of Glab match the five cellular mechanisms described (m.1-m.5). Glab displays anti-inflammatory properties via the inhibition of the NF-κB cascade, and down regulates the expression of COX-2 and iNOS (m.1). Glab also protects LDL against cellular mediated oxidation (m.2), protects PON1 and favors the expression of intracellular antioxidant systems (m.3). Finally, Glab increases energy expenditure and displays anti-adipogenic and hypoglycemic activities (m.4 and 5, Figure 7B). However, Glab and its metabolites have not been tested yet, for activating PPAR-α, or for participating into the Nrf2-Keap-1 signaling cascade. Therefore, these pathways appear to be worthwhile topics of further investigation.
Glab appears to have different biological effects on different cell types (Tables 2 and 3). In their study on anticancer properties of Glab, Kim et al. [97] have highlighted that the mechanism through which Glab exerts these cell-specific effects remains to be elucidated. As a result of this literature survey, it was observed that the multiple biological properties attributed to Glab share at least one common mechanism, likely the regulation of intracellular oxidative stress [130]. This property can be associated with numerous Glab’s effects through the regulation of intracellular pathways (PPAR-γ, NF-κB, PI3K) (Table 6). The principal biological activities attributed to Glab and its 2′-OH isoflavan derivatives have been suggested to be related to their capacity to regulate the level of intracellular ROS [130]. In their short review, Vaya et al. [130] have suggested that 2′, 4′-hydroxyls of Glab and its derivatives are not only required for their antioxidant properties, but also for most of their attributed activities such as ER binding [82], estrogenic-like properties [91, 93], and interactions with cytochrome P450 [77], or even inhibition of tyrosinases [63]. Additionally, Simon et al. have suggested that the prenyl moiety in Glab could be linked to its ERα antagonism in the yeast estrogen assay [92, 95]. Belinky et al. have highlighted the importance of Glab prenyl moiety for hydrophobic interactions, and its integration in either the cellular membrane or the LDL particles, resulting in its membrane antioxidant effect [39, 76]. As such, each aspect of the chemical structure plays an essential role in the bioactivities of Glab (Figure 8).
Table 6.
Glabridin Effects on Intracellular Oxidative Burst and Biological Responses
| Intracellular Antioxidant Activity | Response1 | Biological Effects2 | Ref. |
|---|---|---|---|
| Reduction of intracellular ROS in Raw 264.7 macrophages | Inhibition of NF-κB/Rel activation | Anti-inflammatory | [68] |
| Suppression of MPO activity | Reduction of tissue damages | ||
|
Inhibition of PKC and induction of SOD, CAT and PON2 Protection of mitochondrial respiratory enzymes |
Inhibition of cellular mediated LDL oxidation | Anti-atherogenic | [48] [80] |
| Protection of mitochondrial respiratory Enzymes against peroxidation. | Increase PI3K activity and CREB phosphorylation |
Anti-osteoporotic Promotion of bone recovery |
[100] [98] [97] |
| Up-regulation of antioxidant enzymes SOD1 and GPX4 in MC3T3-E1 Osteoblastic cells |
Up-regulation of PI3K and AKT2 | ||
| Reduction of intracellular ROS induced By UVB radiations in Normal Human Keratinocytes (NHK) | Prevention of DNA damages | Chemo-preventive | [118] |
| Increase of antioxidative markers SOD And glutathione in cerebral tissue of MCAO rats Decrease of MDA level |
Reduction of cerebral ischemia | Neuroprotective | [84] |
Response: Cellular response directly linked to intracellular antioxidant activity.
Biological Effect: General effect observed resulting from the cellular response.
Figure 8. Aspects of the Chemical Structure of Glabridin Involved in Reported Bioactivities.

The structure of Glab could be divided in two major parts. In red, the putative pharmacophore contains the stereogenic center at carbon 3 and the B-ring. The free hydroxyls in C-2′ and C-4′ of the B-ring have been demonstrated to be essential for the expression of its antioxidant effects, mainly for its prevention of LDL oxidation through inhibition of PKC [48] [72]. This B-ring is also involved into binding with the estrogen receptors (ERs) [82], inhibiting cytochrome P450 enzymes such as CYP 3A4 [77], and inhibiting tyrosinase involved in melanogenesis. The 8-prenylation part highlighted in blue contributes to Glab hydrophobicity, and, therefore, to its capacity to incorporate the cell membrane or LDL particles. Simon et al. [92][61] have suggested that prenylation of the A-ring could also take part in its antagonistic effect on ERα in specific tissues and might participate into the expression of its potential phytoSERM properties [93].
Even in light of the above evidence, intracellular antioxidant activities cannot be the sole explanation for the in vivo cellular effects of Glab considering that its concentration in the blood (~13 nM calculated from LFO administration [132]) scarcely reaches effective levels (~μM ; Tables 2–4). Moreover, due to its interaction with P-gp [90], Glab barely reaches the CNS at its effective concentrations. From a general point of view, except for its estrogenic-like activities on peripheral estrogenic tissue, active concentrations of Glab are all in the μM range. Reported bioactive concentrations of Glab clearly differed from one type of biological investigation to another (e.g., dose of Glab used on E0 mice to evaluate its effect on LDL oxidation [20–50 μg/mouse], dose used on BDF1 mice to evaluate the anti-inflammatory properties of Glab [10–50 mg/kg ~0.2–1 mg/mouse]).
Some of the PK parameters of Glab have been investigated; notably its interaction with major cytochromes P450, its intestinal absorption and its bioavailability. Nevertheless, further investigations are required to better define the oral bioavailability of Glab, to prove the existence of potential bioactive metabolites or prodrugs within crude licorice extract, and to obtain a precise profile of P450 interactions. Such information will contribute to a better understanding of in vivo effects of Glab and how they relate to its effective concentrations in vivo. As Glab will be typically consumed by the oral administration of a complex matrix or enriched licorice extract, it will be important to consider structurally related compounds from the extract as potential Glab pro-drugs (e.g., 2′,4′-methylated derivatives). Such congeneric molecules might also participate in the intestinal adsorption of Glab, or act in synergy or additively with Glab.
In an overall summary, this review provides information for an exhaustive chemical characterization and structural dereplication of Glab. The title compound can be readily quantified in a crude extract preparation or even polyherbal mixture by HPLC methods with UV or MS detection. Nevertheless, the current understanding of the molecular and cellular mechanisms of Glab requires expansion in several of the reviewed areas in order to fully support the claim health benefits of this compound. Notably, studies on the estrogenic-like properties of Glab are worth pursuing so as to unravel the molecular mechanism underlying its putative phytoSERM activities. It remains unclear whether Glab itself activates the nuclear receptor PPARs and participates into the Nrf2-Keap-1 signaling cascade. Moreover, reports on the PK properties of Glab are inconsistent and leave ample room for future investigations. In the light of all the above information, Glab can be considered a key species-specific prenylated isoflavan, and is suitable as both chemical and biological marker for the standardization of G. glabra extracts and other licorice preparations.
Acknowledgments
CS wishes particularly to acknowledge Dr. Brent Friesen and Dr. Dejan C. Nikolić for their helpful comments during the preparation of this manuscript. The authors are grateful to Dr. David C. Lankin for the acquisition of the 13C NMR spectrum of glabridin at 225 MHz in the UIC CSB NMR facility, which was funded by NIGMS grant P41 GM068944. CS wishes also to acknowledge Annarita Massarotto from Nature Med (Italy), and Dr. Kevin Spelman from Herb Pharm (Oregon, US) for kindly providing her with samples of G. glabra. CS is particularly grateful to Dr. Julien Herrou for his guidance during the preparation of the figures. This research was supported by NCCAM and ODS of the NIH through grant P50AT000155.
Appendices
Appendix A: NMR Data of Glabridin in DMSO-d6 [40] and CDCl3 [27] Reported in the Literature
The 1H chemical shifts (δ) were expressed in ppm with reference to the residual solvent signal (DMSO-d5, 2.500 ppm and CDCl3, 7.250 ppm relative to the TMS scale), and coupling constants (J) were given in Hertz. In CDCl3 at 300, 400 MHz, resonances of the protons H-5′ and H-6 are overlapping (bold font).
| in DMSO-d6 [40] | In CDCl3 [27] | ||||
|---|---|---|---|---|---|
|
|
|||||
| Position | mult. | δc (75 MHz) | δH (J in Hz) (300 MHz) | δc (100 MHz) | δH (J in Hz) (400 MHz) |
|
| |||||
| 2 pro S | CH2 | 69.84 | 3.92, dd (10.2,10.2) | 70 | 4.01 dd (10.3, 10.3) |
| 2 pro R | 4.23, br d (10.2) | 4.39 ddd (10.3, 3.2, 1.8) | |||
| 3 | CH | 31.01 | 3.29, m | 31.7 | 3.52, m |
| 4 pro S | CH2 | 30.09 | 2.69, dd (15.6, 4.2) | 30.6 | 2.83 ddd (15.7, 5.3, 1.8) |
| 4 pro R | 2.88, dd (15.6, 10.2) | 2.99 dd (15.6, 11.0) | |||
| 5 | CH | 129.22 | 6.81, d (8.2) | 129.2 | 6.81, d (8.2) |
| 6 | CH | 108.21 | 6.27, d (8.2) | 108.7 | 6.33, d (8.2) |
| 7 | C | 151.33 | 151.9 | ||
| 8 | C | 109.15 | 109.15 | ||
| 9 | C | 149.36 | 149.3 | ||
| 10 | C | 114.84 | 114.84 | ||
| 1′ | C | 117.58 | 120.0 | ||
| 2′ (OH) | C | 155.94 | 9.35, s | 154.4 | 7.68 |
| 3′ | CH | 102.63 | 6.32, d (1.3) | 103.1 | 6.46, d (2.2) |
| 4′ (OH) | C | 156.97 | 9.08, s | 155.2 | 7.48 |
| 5′ | CH | 106.41 | 6.18, dd (8.2, 1,3) | 108.0 | 6.39, dd (8.2, 2.2) |
| 6′ | CH | 127.67 | 6.84, d (8.2) | 128.4 | 6.91, d (8.2) |
| 2″ | C | 75.33 | 75.6 | ||
| 3″ | CH | 129.30 | 5.63, d (9.9) | 128.9 | 5.55, d (9.90) |
| 4″ | CH | 116.52 | 6.53, d (9.9) | 116.9 | 6.64, d (9.90) |
| 5″ | CH3 | 27.33 | 1.33, s | 27.6 | 1.40, s |
| 6″ | CH3 | 27.45 | 1.33, s | 27.8 | 1.42, s |
The 1H chemical shifts (δ) were expressed in ppm with reference to the residual solvent signal (DMSO-d5, 2.500 ppm and CDCl3, 7.250 ppm relative to the TMS scale), and coupling constants (J) were given in Hertz. In CDCl3 at 300, 400 MHz, resonances of the protons H-5′ and H-6 are overlapping (bold font).
Appendix B: Comparative 1H NMR Spectra of Glabridin in DMSO-d6 and in CDCl3 Acquired at 900 MHz
±Data were acquired in our laboratory under quantitative conditions at 298 K using a 90° single-pulse experiment (DE = 39.71 μsec, D1 = 60.00 sec, P1 = 10.25 μsec for DMSO-d6 and CDCl3, ds =4, ns = 32) on a Bruker Avance 900 MHz. Off-line data processing were performed using Mnova NMR software package. 1H chemical shifts (δ) were expressed in ppm with reference to the residual solvent signal (DMSO-d5, 2.500 ppm and CDCl3, 7.25 ppm relative to the TMS scale). In the aromatic region, the signals of the protons H-5′ and H-3′ acquired in CDCl3 are inversed in DMSO-d6. From a general point of view, the 1H-NMR fingerprint of glabridin in CDCl3, is totally different from its fingerprint in DMSO-d6.

Appendix C: Infra-Red Spectrum of Glabridin
The IR spectrum was recorded in our laboratory on a FT-IR Nicolet 6700 (Thermo Fisher Scientific) equipped with a DTGS KBr detector (beamsplitter KBr, source IR) using the Omix software. The parameters for the acquisition of this spectrum were as follows: number of scans 32, resolution 4.000, sample gain 8, aperture 100.00, and optical velocity 0.6329. IR ν max in cm−1: 3528, 3351, 2965, 1607, 1519, 1112, 836. This data was in accordance with previously published IR information [40].

Appendix D: HPTLC profile of glabridin and crude extracts from the roots of G. glabra
HPTLC was performed in our laboratory on a nano-adamant silica plate UV254 (0.20 mm, Macherey-Nagel, GmbH & Co.). The different numbers 693, 694, 695, 589 and 686 correspond to different batches of G. glabra roots from which ethanol extracts were prepared (24 hours maceration at room temperature with EtOH, 100%). Each crude extracts were prepared at 10 mg/mL, and 10 μL were spotted on the plate (length = 0.5 cm) using the CAMAG automatic autosampler 4. Glab standard (Glab) was prepared at 0.5 mg/mL and 5 μl were spotted. The elution was performed with a mixture of Chloroform (CHCl3)/MeOH (90/10) on 7 cm from the bottom of the plate, at room temperature. After spraying the plate with sulfuric vanillin (5% v/v) and heating at 110 C during 10 min, Glab appeared as a characteristic pink band (Rf between 0.25 and 0.5). Another HPTLC method has been recently developed by Singh et al. [60], using an eluting solvent composed of Toluene/Dichloromethane/Ethyl-Acetate (1/1/1). The comparative results obtained here highlight the differential concentration of Glab from one batch of roots to another.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.European Food Safety Authority. Scientific Opinion on the safety of “ Glavonoid ® ”, an extract derived from the roots or rootstock of Glycyrrhiza glabra L., as a Novel Food ingredient. EFSA Journal. 2011;9:1–27. [Google Scholar]
- 2.Kishida H, Kitano M. Patent JP 2011001348 A. Formulation for the prevention or alleviation of alcohol drunkenness or hangover. 2011
- 3.Kim GS, Yoo HJ, Kim US, Kim YJ, Yoo SG. Patent KR 1194994 B1. Muscle-building agent containing licorice extracts. 2012
- 4.Shunying J, Wenjia S, Zhijie J. Patent CN 102268107 A. Health care chocolate with good flavor. 2011
- 5.Riccioni G, Speranza L, Pesce M, Cusenza S, D’Orazio N, Glade MJ. Novel phytonutrient contributors to antioxidant protection against cardiovascular disease. Nutrition. 2012;28:605–610. doi: 10.1016/j.nut.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Li Y, Li C, Li D. Natural products and body weight control. N Am J Med Sci. 2011;3:13–19. doi: 10.4297/najms.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veitch NC. Isoflavonoids of the leguminosae. Nat Prod Res. 2009;26:776–802. doi: 10.1039/b616809b. [DOI] [PubMed] [Google Scholar]
- 8.Hassan S, Mathesius U. The role of flavonoids in root-rhizosphere signalling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot. 2012;63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- 9.Boland GM, Donnelly DMX. Isoflavonoids and related compounds. Nat Prod Commun. 1996:241–260. [Google Scholar]
- 10.Aoki T, Akashi T. Flavonoids of leguminous plants: structure, biological activity, and biosynthesis. J Plant Res. 2000;113:475–488. [Google Scholar]
- 11.Akashi T, Koshimizu S, Aoki T, Ayabe SI. Identification of cDNAs encoding pterocarpan reductase involved in isoflavan phytoalexin biosynthesis in Lotus japonicus by EST mining. FEBS Lett. 2006;580:5666–5670. doi: 10.1016/j.febslet.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda M, Mimaki Y, Honda S, Tanaka H, Yokota S, Mae T. Phenolics from Glycyrrhiza glabra roots and their PPAR-gamma ligand-binding activity. Bioorg Med Chem. 2010;18:962–970. doi: 10.1016/j.bmc.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Akashi T, Sasaki K, Aoki T, Ayabe S, Yazaki K. Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin. Plant Physiol. 2009;149:683–693. doi: 10.1104/pp.108.123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi H, Hiraoka N, Ikeshiro Y, Yamamoto H. Organ specific localization of flavonoids in Glycyrrhiza glabra L. Plant Sci. 1996;116:233–238. [Google Scholar]
- 15.Hayashi H, Hattori S, Inoue K, Khodzhimatov O, Ashurmetov O, Ito M, et al. Field survey of Glycyrrhiza plants in Central Asia (3). Chemical characterization of G. glabra collected in Uzbekistan. Chem Pharm Bull. 2003;51:1338–1340. doi: 10.1248/cpb.51.1338. [DOI] [PubMed] [Google Scholar]
- 16.Kondo K, Hiba MS, Akamura RN, Orota TM, Hoyama YS. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol Pharm Bull. 2007;30:1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa K, Kishida H, Arai N, Nishiyama T, Mae T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK-A(y) mice. Biol Pharm Bull. 2004;27:1775–1778. doi: 10.1248/bpb.27.1775. [DOI] [PubMed] [Google Scholar]
- 18.Tian M, Yan H. Extraction of glycyrrhizic acid and glabridin from licorice. Int J Mol Sci. 2008;9:571–577. doi: 10.3390/ijms9040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CHO Y, RYU J. Supercritical fluid extraction of glabridin from Glycyrrhiza glabra. Proceedings of the 4th International Conference on Separation Science and Technology; 2004. pp. 334–337. [Google Scholar]
- 20.Li X, Guo R, Zhang X, Li X. Extraction of glabridin using imidazolium-based ionic liquids. Sep Purif Technol. 2012;88:146–150. [Google Scholar]
- 21.Yan H, Tian M, Row KH. Selective solid-phase extraction of glabridin from licorice root using molecularly imprinted polymer. Sep Sci Technol. 2009;44:359–369. [Google Scholar]
- 22.Xu Y, Yuan Q, Hou X. Preparative separation of glabridin from Glycyrrhiza glabra L. extracts with macroporous resins. Sep Sci Technol. 2009;44:3717–3734. [Google Scholar]
- 23.Ahn J, Lee H, Jang J, Kim S, Ha T. Anti-obesity effects of glabridin-rich supercritical carbon dioxide extract of licorice in high-fat-fed obese mice. Food Chem Toxicol. 2013;51:439–445. doi: 10.1016/j.fct.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee M, Bhaskaran N, Srinath R, Shivaprasad HN, Allan JJ, Shekhar D, et al. Anti-ulcer and antioxidant activity of GutGard. Indian J Exp Biol. 2010;48:269–274. [PubMed] [Google Scholar]
- 25.Raveendra KR, Jayachandra, Srinivasa V, Sushma KR, Allan JJ, Goudar KS, et al. An extract of Glycyrrhiza glabra (GutGard) alleviates symptoms of functional dyspepsia: a randomized, double-blind, placebo-controlled study. Evid Based Complement Alternat Med. 2012;2012:1–9. doi: 10.1155/2012/216970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian M, Yan H, Row KH. Simultaneous extraction and separation of liquiritin, glycyrrhizic acid, and glabridin from licorice root with analytical and preparative chromatography. Biotechnol and Bioprocess Eng. 2009;13:671–676. doi: 10.1007/s12257-008-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinoshita T, Kajiyama K, Hiraga Y. Isoflavan derivatives from Glycyrrhiza glabra (licorice) Heterocycles. 1996;43:581–588. [Google Scholar]
- 28.Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 29.Saitoh T, Kinoshita T, Shibata S. New isoflavan isolated from licorice root. Chem Pharm Bull. 1976;24:752–755. [Google Scholar]
- 30.Cui YM, Ao MZ, Li W, Yu LJ. Effect of glabridin from Glycyrrhiza glabra on learning and memory in mice. Planta Med. 2008;74:377–380. doi: 10.1055/s-2008-1034319. [DOI] [PubMed] [Google Scholar]
- 31.Mitscher L, Park Y, Clark D. Antimicrobial agents from higher plants. Antimicrobial isoflavanoids and related substances from Glycyrrhiza glabra L. var. typica. J Nat Prod. 1980:259–269. doi: 10.1021/np50008a004. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Muhebuli A, Bahargul K, He Q. Preparation technology of isoflavan glabridin from Glycyrrhiza glabra L. Xinjiang Yike Daxue Xuebao. 2007;30:692–694. [Google Scholar]
- 33.Lv J, Liang H, Yuan Q, Xu Y, Wang T. Preparative purification of the major flavonoid glabridin from licorice roots by solid phase extraction and preparative high performance liquid chromatography. Sep Sci Technol. 2010;45:1104–1111. [Google Scholar]
- 34.Dong W, Guo J, Wen H, Liu D, Xia K. U.S. Patent CN 101830906 A. Separation and purification method of high-purity glabridin. 2010
- 35.Changying H. Preparation of high purity glabridin from Glycyrrhiza glabra. Patent CN 101735233 A. 2010
- 36.Liuhua S. Patent CN 102250107 A. Method for preparing glabridin. 2011
- 37.Zhu Y, Sun D. Patent CN 1680386 A. Method for producing high-purity glabridin by solvent extraction, adsorption and recrystallization. 2005
- 38.Kuang J. Patent CN 1616461 A. Method for producing glabridin product. 2005
- 39.Belinky PA, Aviram M, Mahmood S, Vaya J. Structural aspects of the inhibitory effect of glabridin on LDL oxidation. Free Radic Biol Med. 1998;24:1419–1429. doi: 10.1016/s0891-5849(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 40.Jirawattanapong W, Saifah E, Patarapanich C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch Pharm Res. 2009;32:647–654. doi: 10.1007/s12272-009-1501-x. [DOI] [PubMed] [Google Scholar]
- 41.Tantishaiyakul V, Suknuntha K, Saithong S, Pakawatchai C. Glabridin. Acta Crystallogr Sect E: Struct Rep Online. 2012;68:o3501–o3501. doi: 10.1107/S1600536812048647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M, Kim S, Kim Y, Han J. Absolute configurations of (±) glabridin enantiomers. Configurations. 2009;30:415–418. [Google Scholar]
- 43.Yoo S, Nahm K. Facile and efficient synthesis of (±) glabridin. Bull Korean Chem Soc. 2007;28:481–484. [Google Scholar]
- 44.Yoo S-K, Kang H-K, Kang K-S, Nahm K, You SW. Patent WO 2005037815 A1. A manufacturing process of isoflavan or isoflavene derivatives. 2005
- 45.Koyakumaru K, Ueyama S. Patent JP 2006008604 A. Preparation of isoflavans as peroxisome proliferator-activated receptor ligands. 2006
- 46.Koyakumaru K. Patent JP 2006008606 A. Optical resolution of isoflavans as peroxisome proliferator-activated receptor ligands. 2006
- 47.Ao M, Shi Y, Cui Y, Guo W, Wang J, Longjiang Y. Factors influencing glabridin stability. Nat Prod Commun. 2010;5:1907–1912. [PubMed] [Google Scholar]
- 48.Rosenblat M, Belinky P, Vaya J, Levy R. Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein. J Biol Chem. 1999;274:13790–13799. doi: 10.1074/jbc.274.20.13790. [DOI] [PubMed] [Google Scholar]
- 49.Nakahara T. Patent JP 2007112765 A. UV absorbers containing glabridin glycosides. 2007
- 50.Murakami T, Miyoshi S, Kishida N, Dohi K. Patent JP 2004277309 A. Glabridin glycosides, their preparation, and their use for tyrosinase inhibitors and topical formulations. 2004
- 51.Schoenrock U, Untiedt S, Kux U, Inoue K. Patent De 19615576 A1. Application of glabridin mono-and/or diglucosides as anti-irritants in cosmetic and topical dermatological preparations. 1997
- 52.Nishio H. Patent JP 06256353 A. Preparation of glabridin derivatives with activity of lightening dark skin color. 1994
- 53.Ikemoto T, Oota M. Patent JP 05320152 A. Preparation of glabridin diesters as bactericides and melanin formation inhibitors. 1993
- 54.Jirawattanapong W, Saifah E, Patarapanich C. A validated stability-indicating HPLC method for analysis of glabridin prodrugs in hydrolysis studies. Drug Discov Ther. 2009;3:97–103. [PubMed] [Google Scholar]
- 55.Hsieh CW, Li PH, Lu IC, Wang TH. Preparing glabridin-in-water nanoemulsions by high pressure homogenization with response surface methodology. J Oleo Sci. 2012;61:483–489. doi: 10.5650/jos.61.483. [DOI] [PubMed] [Google Scholar]
- 56.Park Y, Park HJ, Jaegwan L. Stabilisation of glabridin by chitosan nano-complex. J Korean Soc Appl Biol Chem. 2012;55:457–462. [Google Scholar]
- 57.Deshmukh K, Poddar SS. Tyrosinase inhibitor-loaded microsponge drug delivery system: new approach for hyperpigmentation disorders. J Microencapsul. 2012;29:559–568. doi: 10.3109/02652048.2012.668955. [DOI] [PubMed] [Google Scholar]
- 58.Kamal Y, Singh M. Rapid RP-HPLC method for the quantification of glabridin in crude drug and in polyherbal formulation. J Chromatogr Sci. 2012;50:779–784. doi: 10.1093/chromsci/bms063. [DOI] [PubMed] [Google Scholar]
- 59.Aoki F, Nakagawa K, Tanaka A, Matsuzaki K, Arai N, Mae T. Determination of glabridin in human plasma by solid-phase extraction and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;828:70–74. doi: 10.1016/j.jchromb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Singh M, Kamal YTK, Tamboli ET, Parveen R, Ansari SH, Ahmad S. Glabridin, a stable flavonoid of Glycyrrhiza glabra: HPTLC analysis of the traditional formulation. J Planar Chromatogr-Mod TLC. 2013;26:267–273. [Google Scholar]
- 61.Simons R, Gruppen H, Bovee T. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs) Food Funct. 2012;3:810–827. doi: 10.1039/c2fo10290k. [DOI] [PubMed] [Google Scholar]
- 62.Jungbauer A, Medjakovic S. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998;11:355–361. doi: 10.1111/j.1600-0749.1998.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 64.Chandrasekaran CV, Deepak HB, Thiyagarajan P, Kathiresan S, Sangli GK, Deepak M, et al. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 2011;18:278–284. doi: 10.1016/j.phymed.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Park SH, Kang JS, Yoon YD, Lee K, Kim K, Lee KH, et al. Glabridin inhibits Lipopolysaccharide-induced activation of a microglial cell line, BV-2, by blocking NF-kB and AP-1. Phytother Res. 2010;34:29–34. doi: 10.1002/ptr.2872. [DOI] [PubMed] [Google Scholar]
- 66.Kim JY, Kang JS, Kim HM, Ryu HS, Kim HS, Lee HK, et al. Inhibition of bone marrow-derived dendritic cell maturation by glabridin. Int Immunopharmacol. 2010;10:1185–1193. doi: 10.1016/j.intimp.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 67.Kwon HS, Oh SM, Kim JK. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2008;151:165–173. doi: 10.1111/j.1365-2249.2007.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang JS, Yoon YD, Cho IJ, Han MH, Lee CW, Park S, et al. Glabridin, an isoflavan from licorice root, inhibits Inducible nitric-oxide synthase expression and improves survival of mice in experimental model of septic shock. J Pharmacol Exp Ther. 2005;312:1187–1194. doi: 10.1124/jpet.104.077107. [DOI] [PubMed] [Google Scholar]
- 69.Thiyagarajan P, Chandrasekaran CV, Deepak HB, Agarwal A. Modulation of lipopolysaccharide-induced pro-inflammatory mediators by an extract of Glycyrrhiza glabra and its phytoconstituents. Inflammopharmacology. 2011;19:235–241. doi: 10.1007/s10787-011-0080-x. [DOI] [PubMed] [Google Scholar]
- 70.Kim HM, Kang JS, Park S-K, Lee K, Kim JY, Han SB, et al. U.S. Patent WO2009069887 A1. A Pharmaceutical composition comprising glabridin or glabridin derivatives for suppression of dendritic cell maturation. 2009
- 71.Ito C, Oi N, Hashimoto T, Nakabayashi H, Aoki F, Tominaga Y, et al. Absorption of dietary licorice isoflavan glabridin to blood circulation in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:358–365. doi: 10.3177/jnsv.53.358. [DOI] [PubMed] [Google Scholar]
- 72.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000;33:S85–97. [PubMed] [Google Scholar]
- 73.Levitan I. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13:39–75. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 1995;76:18–23. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 75.Fuhrman B, Buch S, Vaya J, Belinky Pa, Coleman R, Hayek T, et al. Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: in vitro and ex vivo studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 1997;66:267–275. doi: 10.1093/ajcn/66.2.267. [DOI] [PubMed] [Google Scholar]
- 76.Belinky PA, Aviram M, Fuhrman B, Rosenblat M, Vaya J. The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis. 1998;137:49–61. doi: 10.1016/s0021-9150(97)00251-7. [DOI] [PubMed] [Google Scholar]
- 77.Kent U, Aviram M, Rosenblat M, Hollenberg PF. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab Dispos. 2002;30:709–715. doi: 10.1124/dmd.30.6.709. [DOI] [PubMed] [Google Scholar]
- 78.Carmeli E, Fogelman Y. Antioxidant effect of polyphenolic glabridin on LDL oxidation. Toxicol Ind Health. 2009;25:321–324. doi: 10.1177/0748233709103034. [DOI] [PubMed] [Google Scholar]
- 79.Rosenblat M, Aviram M. Paraoxonases role in the prevention of cardiovascular diseases. BioFactors. 2008;35:98–104. doi: 10.1002/biof.16. [DOI] [PubMed] [Google Scholar]
- 80.Yehuda I, Madar Z, Szuchman-Sapir A, Tamir S. Glabridin, a phytoestrogen from licorice root, up-regulates manganese superoxide dismutase, catalase and paraoxonase 2 under glucose stress. Phytother Res. 2011;25:659–667. doi: 10.1002/ptr.3318. [DOI] [PubMed] [Google Scholar]
- 81.Atrahimovich D, Vaya J, Tavori H, Khatib S. Glabridin protects paraoxonase 1 from linoleic acid hydroperoxide inhibition via specific interaction: a fluorescence-quenching study. J Agric Food Chem. 2012;60:3679–3685. doi: 10.1021/jf2046009. [DOI] [PubMed] [Google Scholar]
- 82.Somjen D, Knoll E, Vaya J, Stern N, Tamir S. Estrogen-like activity of licorice root constituents: glabridin and glabrene, in vascular tissues in vitro and in vivo. J Steroid Biochem Mol Biol. 2004;91:147–155. doi: 10.1016/j.jsbmb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Kang JS, Yoon YD, Han MH, Han S, Lee K, Lee KH, et al. Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway : implications of Akt, extracellular signal-regulated. Mol Pharmacol. 2006;69:941–949. doi: 10.1124/mol.105.017442. [DOI] [PubMed] [Google Scholar]
- 84.Yu X, Xue C, Zhou Z, Li C, Du Y. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice) Life Sci. 2008;82:68–78. doi: 10.1016/j.lfs.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Roth KA, Shacka JJ. Apoptosis in neurodegenerative disease. Encyclopedia of Neuroscience. 2009:531–537. [Google Scholar]
- 86.Hasanein P. Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiol Hung. 2011;98:221–230. doi: 10.1556/APhysiol.98.2011.2.14. [DOI] [PubMed] [Google Scholar]
- 87.Lines TC. Patent WO 2006091988 A1. Composition for treating mental health disorders. 2006
- 88.Ofir R, Tamir S, Khatib S, Vaya J. Inhibition of serotonin re-uptake by licorice constituents. J Mol Neurosci. 2003;20:135–140. doi: 10.1385/JMN:20:2:135. [DOI] [PubMed] [Google Scholar]
- 89.Fuller RW. Basic advances in serotonin pharmacology. J Clin Psychiatry. 1992;53:36–45. [PubMed] [Google Scholar]
- 90.Yu X, Lin S, Zhou Z, Chen X, Liang J, Yu X, et al. Role of P-glycoprotein in limiting the brain penetration of glabridin, an active isoflavan from the root of Glycyrrhiza glabra. Pharm Res. 2007;24:1668–1690. doi: 10.1007/s11095-007-9297-1. [DOI] [PubMed] [Google Scholar]
- 91.Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, Kaye A, et al. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000;60:5704–5709. [PubMed] [Google Scholar]
- 92.Simons R, Vincken JP, Mol LAM, The SAM, Bovee TFH, Luijendijk TJC, et al. Agonistic and antagonistic estrogens in licorice root (Glycyrrhiza glabra) Anal Bioanal Chem. 2011;401:305–313. doi: 10.1007/s00216-011-5061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Somjen D, Katzburg S, Vaya J, Kaye AM, Hendel D, Posner GH, et al. Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. J Steroid Biochem Mol Biol. 2004;91:241–246. doi: 10.1016/j.jsbmb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Vaya J, Tamir S. U.S. Patent WO01/32191 A2. Phytoestrogenic pharmaceutical preparations. 2001
- 95.Simons R, Gruppen H, Bovee T. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs) Food & Function. 2012;3:810–827. doi: 10.1039/c2fo10290k. [DOI] [PubMed] [Google Scholar]
- 96.Choi EM. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2005;70:363–368. doi: 10.1016/j.bcp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 97.Kim HS, Suh KS, Ko A, Sul D, Choi D, Lee SK, et al. The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. Int J Mol Med. 2013;31:243–251. doi: 10.3892/ijmm.2012.1172. [DOI] [PubMed] [Google Scholar]
- 98.Choi EM. Glabridin protects osteoblastic MC3T3-E1 cells against antimycin A induced cytotoxicity. Chem Biol Interact. 2011;193:71–78. doi: 10.1016/j.cbi.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 99.Somjen D, Katzburg S, Sharon O, Posner GH, Jaccard N, Hendel D, et al. Mutual interaction of special phytoestrogenic compounds, their synthetic carboxy-derivatives and the less-calcemic vitamin D analog activities in human derived female cultured osteoblasts. J Steroid Biochem Mol Biol. 2011;127:351–357. doi: 10.1016/j.jsbmb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Haraguchi H, Yoshida N, Ishikawa H, Tamura Y, Mizutani K, Kinoshita T. Protection of mitochondrial functions against oxidative stresses by isoflavans from Glycyrrhiza glabra. J Pharm Pharmacol. 2000;52:219–223. doi: 10.1211/0022357001773724. [DOI] [PubMed] [Google Scholar]
- 101.Guntur AR, Rosen CJ. New insights into osteoblasts and their role in bone formation: the central role of PI3 kinase. J Endocrinol. 2011;211:123–130. doi: 10.1530/JOE-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim HS, Suh KS, Sul D, Kim BJ, Lee SK, Jung WW. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29:169–177. doi: 10.3892/ijmm.2011.822. [DOI] [PubMed] [Google Scholar]
- 103.Viollet B, Andreelli F. AMP-Activated Protein Kinase and Metabolic Control. Handb Exp Pharmacol. 2011;203:303–330. doi: 10.1007/978-3-642-17214-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 105.Cristancho AG, Lazar Ma. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem. 2007;71:206–214. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 107.Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jo H, et al. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res. 2012;53:1277–1286. doi: 10.1194/jlr.M022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shang H, Cao S, Wang J, Zheng H, Putheti R. Glabridin from Chinese herb licorice inhibits fatigue in mice. Afr J Tradit Complement Altern Med. 2010;7:17–23. doi: 10.4314/ajtcam.v7i1.57225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu F, Jin Z, Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7:1278–1282. doi: 10.3892/mmr.2013.1330. [DOI] [PubMed] [Google Scholar]
- 110.Birari R, Gupta S, Mohan C, Bhutani K. Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: Experimental and computational studies. Phytomedicine. 2011;18:795–801. doi: 10.1016/j.phymed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Park MG, Yoo S. U.S. Patent WO 2007058480 A1. Composition having effect on treatment and prevention of diseases syndrome treatment with glabridin. 2007
- 112.Hayami T, Kishida H, Arai N, Yokota S, Hamada K. Patent WO 2005110400 A1. Lipase inhibitor, cholesterol esterase inhibitor, neutral fat absorption inhibitor, cholesterol absorption inhibitor and cholesterol ester absorption inhibitor. 2005
- 113.Arai N, Kishida H, Ikehara T, Mae T. U.S. Patent WO 2005011672 A1. Processed fat composition for preventing/ameliorating lifestyle-related diseases. 2005
- 114.Tsai Y-M, Yang C-J, Hsu Y-L, Wu L-Y, Tsai Y-C, Hung J-Y, et al. Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther. 2011;10:341–349. doi: 10.1177/1534735410384860. [DOI] [PubMed] [Google Scholar]
- 115.Hsu YL, Wu LY, Hou MF, Tsai EM, Lee JN, Liang HL, et al. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res. 2011;55:318–327. doi: 10.1002/mnfr.201000148. [DOI] [PubMed] [Google Scholar]
- 116.Nabekura T, Yamaki T, Ueno K, Kitagawa S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother Pharmacol. 2008;62:867–873. doi: 10.1007/s00280-007-0676-4. [DOI] [PubMed] [Google Scholar]
- 117.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–2869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verrati E, Rossi T, Giudice S, Benassi L, Bertazzoni G, Morini D, et al. 18β-glycyrrhetinic acid and glabridin prevent oxidative DNA fragmentation in UVB-irradiated human keratinocyte cultures. Anticancer Res. 2011;31:2209–2216. [PubMed] [Google Scholar]
- 119.Fukai T, Satoh K, Nomura T, Sakagami H. Preliminary evaluation of antinephritis and radical scavenging activities of glabridin from Glycyrrhiza glabra. Fitoterapia. 2003;74:624–629. doi: 10.1016/s0367-326x(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 120.Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int J Cosmet Sci. 2011;33:210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 121.Yamauchi K. Isolation, identification and tyrosinase inhibitory activities of the extractives from Allamanda cathartica. Nat Resources. 2011;02:167–172. [Google Scholar]
- 122.Majeed B, Seshadri K, Gangadharan K, Subbalakshmi P. Patent US 20040121031 A1. Novel topical skin care and nutraceutical applications of glabridin or extracts containing a defined amount of gabridin. 2004
- 123.Weinberger GI, Nagel HR, Brown RA. Patent WO 2012/177433 A1. Topical compositions for the treatment of dermatological disorders. 2012
- 124.Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2002;73:536–539. doi: 10.1016/s0367-326x(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 125.Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of Licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of Licorice phenolics on Methicillin-Resistant Staphylococcus aureus. Chem Pharm Bull. 2000;48:1286–1292. doi: 10.1248/cpb.48.1286. [DOI] [PubMed] [Google Scholar]
- 126.Messier C, Grenier D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses. 2011;54:e801–806. doi: 10.1111/j.1439-0507.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- 127.Fatima A, Gupta VK, Luqman S, Negi AS, Kumar JK, Shanker K, et al. Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother Res. 2009;1193:1190–1193. doi: 10.1002/ptr.2726. [DOI] [PubMed] [Google Scholar]
- 128.Asha MK, Debraj D, Prashanth D, Edwin JR, Srikanth HS, Muruganantham N, et al. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J Ethnopharmacol. 2013;145:581–586. doi: 10.1016/j.jep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 129.Ono H, Yamamoto S, Miyagawa H. Patent JP 2006298877 A. Plant disease-preventing agent extracted from licorice and agrochemicals and fertilizers containing glabridin. 2006
- 130.Vaya J, Somjen D, Tamir S. The role of isoflavan’s 2′-hydroxyl in diverse biological activities. XI Biennial Meeting of the Society for Free Radical Research International; 2002. pp. 567–574. [Google Scholar]
- 131.Cao J, Chen X, Liang J, Yu X, Xu A, Chan E, et al. Role of P-glycoprotein in the intestinal absorption of Glabridin, an active flavonoid from the root of Glycyrrhiza glabra. Drug Metab Dispos. 2007;35:539–553. doi: 10.1124/dmd.106.010801. [DOI] [PubMed] [Google Scholar]
- 132.Aoki F, Nakagawa K, Kitano M, Ikematsu H, Nakamura K, Yokota S, et al. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J Am Coll Nutr. 2007;26:209–218. doi: 10.1080/07315724.2007.10719603. [DOI] [PubMed] [Google Scholar]
- 133.Pauli GF, Chen SN, Friesen JB, McAlpine JB, Jaki BU. Analysis and purification of bioactive natural products: the AnaPurNa study. J Nat Prod. 2012;75:1243–1255. doi: 10.1021/np300066q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]






