electron shell
Our editors will review what you’ve submitted and determine whether to revise the article.
- Related Topics:
- orbital
- quantum number
- subshell
- valence shell
- closed shell
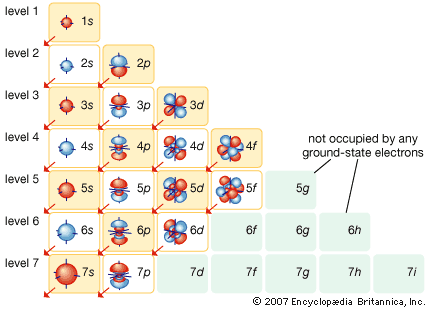
electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each allowed electron orbit is assigned a quantum number n that runs from 1 (for the orbit closest to the nucleus) to infinity (for orbits very far from the nucleus). All the orbitals that have the same value of n make up a shell. Inside each shell there are subshells corresponding to different rates of rotation and orientation of orbitals and the spin directions of the electrons. In general, the farther away from the nucleus (or the higher the value of n) a shell is, the more subshells it will have. The n = 1 electron shell can hold two electrons; n = 2, 8; n = 3, 18; and n = 4, 32.