carbon
Our editors will review what you’ve submitted and determine whether to revise the article.
- Live Science - Carbon: Facts about an element that is a key ingredient for life on Earth
- Purdue University - Chemical Education Division Groups - The Inorganic Chemistry of Carbon
- National Center for Biotechnology Information - PubChem - Carbon
- LSU College of Sciences - Department of Physics and Astronomy - An Overview of Synthetic Diamond and Diamond-like Carbon
- Jefferson Lab - Carbon
- Lenntech - Carbon - C
- Chemistry LibreTexts - Carbon and Fireworks
- Khan Academy - Carbon and hydrocarbons
- Royal Society of Chemistry - Carbon
- Chemicool - Carbon
- University of Hawaiʻi Pressbooks - Carbon
carbon (C), nonmetallic chemical element in Group 14 (IVa) of the periodic table. Although widely distributed in nature, carbon is not particularly plentiful—it makes up only about 0.025 percent of Earth’s crust—yet it forms more compounds than all the other elements combined. In 1961 the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured. Carbon-14, which is radioactive, is the isotope used in radiocarbon dating and radiolabeling.
| atomic number | 6 |
|---|---|
| atomic weight | 12.0096 to 12.0116 |
| melting point | 3,550 °C (6,420 °F) |
| boiling point | 4,827 °C (8,721 °F) |
| density | |
| diamond | 3.52 g/cm3 |
| graphite | 2.25 g/cm3 |
| amorphous | 1.9 g/cm3 |
| oxidation states | +2, +3, +4 |
| electron configuration | 1s22s22p2 |
Properties and uses
On a weight basis, carbon is 19th in order of elemental abundance in Earth’s crust, and there are estimated to be 3.5 times as many carbon atoms as silicon atoms in the universe. Only hydrogen, helium, oxygen, neon, and nitrogen are atomically more abundant in the cosmos than carbon. Carbon is the cosmic product of the “burning” of helium, in which three helium nuclei, atomic weight 4, fuse to produce a carbon nucleus, atomic weight 12.
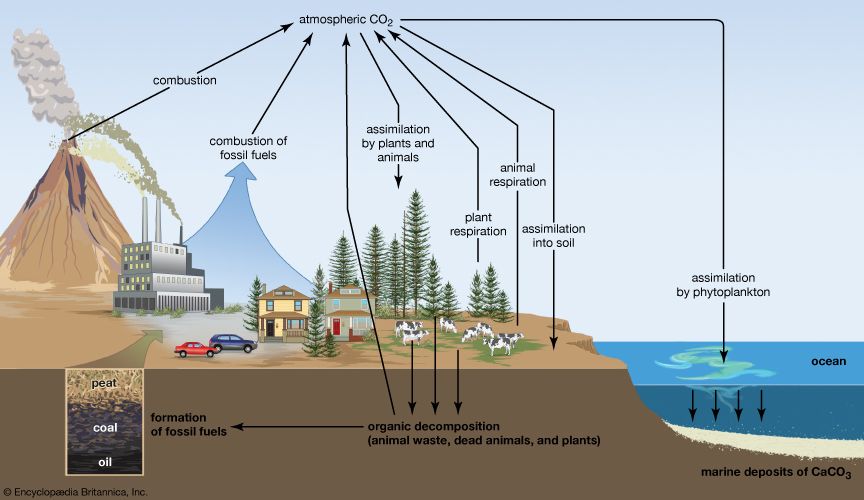
In the crust of Earth, elemental carbon is a minor component. However, carbon compounds (i.e., carbonates of magnesium and calcium) form common minerals (e.g., magnesite, dolomite, marble, or limestone). Coral and the shells of oysters and clams are primarily calcium carbonate. Carbon is widely distributed as coal and in the organic compounds that constitute petroleum, natural gas, and all plant and animal tissue. A natural sequence of chemical reactions called the carbon cycle—involving conversion of atmospheric carbon dioxide to carbohydrates by photosynthesis in plants, the consumption of these carbohydrates by animals and oxidation of them through metabolism to produce carbon dioxide and other products, and the return of carbon dioxide to the atmosphere—is one of the most important of all biological processes.
Carbon as an element was discovered by the first person to handle charcoal from fire. Thus, together with sulfur, iron, tin, lead, copper, mercury, silver, and gold, carbon was one of the small group of elements well known in the ancient world. Modern carbon chemistry dates from the development of coals, petroleum, and natural gas as fuels and from the elucidation of synthetic organic chemistry, both substantially developed since the 1800s.
Elemental carbon exists in several forms, each of which has its own physical characteristics. Two of its well-defined forms, diamond and graphite, are crystalline in structure, but they differ in physical properties because the arrangements of the atoms in their structures are dissimilar. A third form, called fullerene, consists of a variety of molecules composed entirely of carbon. Spheroidal, closed-cage fullerenes are called buckerminsterfullerenes, or “buckyballs,” and cylindrical fullerenes are called nanotubes. A fourth form, called Q-carbon, is crystalline and magnetic. Yet another form, called amorphous carbon, has no crystalline structure. Other forms—such as carbon black, charcoal, lampblack, coal, and coke—are sometimes called amorphous, but X-ray examination has revealed that these substances do possess a low degree of crystallinity. Diamond and graphite occur naturally on Earth, and they also can be produced synthetically; they are chemically inert but do combine with oxygen at high temperatures, just as amorphous carbon does. Fullerene was serendipitously discovered in 1985 as a synthetic product in the course of laboratory experiments to simulate the chemistry in the atmosphere of giant stars. It was later found to occur naturally in tiny amounts on Earth and in meteorites. Q-carbon is also synthetic, but scientists have speculated that it could form within the hot environments of some planetary cores.

The word carbon probably derives from the Latin carbo, meaning variously “coal,” “charcoal,” “ember.” The term diamond, a corruption of the Greek word adamas, “the invincible,” aptly describes the permanence of this crystallized form of carbon, just as graphite, the name for the other crystal form of carbon, derived from the Greek verb graphein, “to write,” reflects its property of leaving a dark mark when rubbed on a surface. Before the discovery in 1779 that graphite when burned in air forms carbon dioxide, graphite was confused with both the metal lead and a superficially similar substance, the mineral molybdenite.
Pure diamond is the hardest naturally occurring substance known and is a poor conductor of electricity. Graphite, on the other hand, is a soft slippery solid that is a good conductor of both heat and electricity. Carbon as diamond is the most expensive and brilliant of all the natural gemstones and the hardest of the naturally occurring abrasives. Graphite is used as a lubricant. In microcrystalline and nearly amorphous form, it is used as a black pigment, as an adsorbent, as a fuel, as a filler for rubber, and, mixed with clay, as the “lead” of pencils. Because it conducts electricity but does not melt, graphite is also used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted. Molecules of fullerene show promise in a range of applications, including high-tensile-strength materials, unique electronic and energy-storage devices, and safe encapsulation of flammable gases, such as hydrogen. Q-carbon, which is created by rapidly cooling a sample of elemental carbon whose temperature has been raised to 4,000 K (3,727 °C [6,740 °F]), is harder than diamond, and it can be used to manufacture diamond structures (such as diamond films and microneedles) within its matrix. Elemental carbon is nontoxic.
Each of the “amorphous” forms of carbon has its own specific character, and, hence, each has its own particular applications. All are products of oxidation and other forms of decomposition of organic compounds. Coal and coke, for example, are used extensively as fuels. Charcoal is used as an absorptive and filtering agent and as a fuel and was once widely used as an ingredient in gunpowder. (Coals are elemental carbon mixed with varying amounts of carbon compounds. Coke and charcoal are nearly pure carbon.) In addition to its uses in making inks and paints, carbon black is added to the rubber used in tires to improve its wearing qualities. Bone black, or animal charcoal, can adsorb gases and colouring matter from many other materials.
Carbon, either elemental or combined, is usually determined quantitatively by conversion to carbon dioxide gas, which can then be absorbed by other chemicals to give either a weighable product or a solution with acidic properties that can be titrated.
Production of elemental carbon
Until 1955 all diamonds were obtained from natural deposits, most significant in southern Africa but occurring also in Brazil, Venezuela, Guyana, and Siberia. The single known source in the United States, in Arkansas, has no commercial importance; nor is India, once a source of fine diamonds, a significant present-day supplier. The primary source of diamonds is a soft bluish peridotic rock called kimberlite (after the famous deposit at Kimberley, South Africa), found in volcanic structures called pipes, but many diamonds occur in alluvial deposits presumably resulting from the weathering of primary sources. Isolated finds around the world in regions where no sources are indicated have not been uncommon.
Natural deposits are worked by crushing, by gravity and flotation separations, and by removal of diamonds by their adherence to a layer of grease on a suitable table. The following products result: (1) diamond proper—distorted cubic crystalline gem-quality stones varying from colourless to red, pink, blue, green, or yellow; (2) bort—minute dark crystals of abrasive but not gem quality; (3) ballas—randomly oriented crystals of abrasive quality; (4) macles—triangular pillow-shaped crystals that are industrially useful; and (5) carbonado—mixed diamond–graphite crystallites containing other impurities.
The successful laboratory conversion of graphite to diamond was made in 1955. The procedure involved the simultaneous use of extremely high pressure and temperature with iron as a solvent or catalyst. Subsequently, chromium, manganese, cobalt, nickel, and tantalum were substituted for iron. Synthetic diamonds are now manufactured in several countries and are being used increasingly in place of natural materials as industrial abrasives.
Graphite occurs naturally in many areas, the deposits of major importance being in China, India, Brazil, Turkey, Mexico, Canada, Russia, and Madagascar. Both surface- and deep-mining techniques are used, followed by flotation, but the major portion of commercial graphite is produced by heating petroleum coke in an electric furnace. A better crystallized form, known as pyrolytic graphite, is obtained from the decomposition of low-molecular-weight hydrocarbons by heat. Graphite fibres of considerable tensile strength are obtained by carbonizing natural and synthetic organic fibres.
Carbon products are obtained by heating coal (to give coke), natural gas (to give blacks), or carbonaceous material of vegetable or animal origin, such as wood or bone (to give charcoal), at elevated temperatures in the presence of insufficient oxygen to allow combustion. The volatile by-products are recovered and used separately.