Abstract
This study evaluated the efficacy and tolerability of topical timolol, 0.5%, gel as a treatment of paronychia and pseudopyogenic granuloma induced by antineoplastic agents.
Paronychia and pseudopyogenic granuloma (pseudo-PG) are relatively common and difficult-to-manage adverse effects in patients undergoing treatment with epidermal growth factor receptor inhibitors and capecitabine. Recently, Piraccini et al reported the successful treatment of chemotherapy-induced fingernail PGs with topical propranolol, 1%, cream. The aim of this study was to evaluate the efficacy and tolerability of topical timolol, 0.5%, gel as a treatment of paronychia and pseudo-PG induced by antineoplastic agents.
Methods
We included 10 patients who were referred to our department with paronychia and/or periungual pseudo-PG as an adverse effect of epidermal growth factor receptor inhibitor or capecitabine from September 1, 2016, to May 31, 2017. The protocol was submitted to and approved by the institutional review board of Hospital de la Santa Creu i Sant Pau, and patients gave their oral informed consent to participate in the study. All data were deidentified.
Patients’ demographic characteristics, lesion localization (fingernails, toenails, or both), number of lesions, duration of antineoplastic treatment before the appearance of lesions, and other skin manifestations were also recorded. All lesions were treated with topical timolol, 0.5%, gel (Timogel) twice daily under occlusion for 1 month. Response to treatment was assessed by clinical examination and photographic control at baseline and after 1 month of treatment and classified as complete response (disappearance of the lesion, absent pain, and/or bleeding), partial response (improvement in at least 1 of these 3 items), or lack of response.
Results
Of the 10 patients in the study, 5 were women and 5 were men (mean [SD] age, 66.4 [10.1] years; age range, 51-78 years). The baseline characteristics of the patients are summarized in the Table. The mean (SD) antineoplastic treatment duration was 3.8 (3.2) months (range, 1-12 months). Four patients were treated with panitumumab, 4 with cetuximab, 1 with erlotinib, and 1 with capecitabine. Seven patients were also treated with other antineoplastic agents.
Table. Clinical Characteristics and Treatment of the Patients Observed.
| Patient No./Sex/Age, y | Primary Neoplasia | Lesion Localization (No. of Lesions) |
Neoplasia Treatment | Co-treatment | Antineoplastic Treatment Duration Before Lesions, mo | Other Toxic Effects | Timolol Response Evaluation |
|---|---|---|---|---|---|---|---|
| 1/M/60s | Pyriform sinus carcinoma, stage IV | Fingernail (1 PR and 1 PG) | Cetuximab | Cyclobutane dicarboxylic acid or carboplatin with fluorouracil | 4 | PPE | Complete |
| 2/M/50s | Colon ADK, stage IV | Toenail (2 PR and 2 PG) | Panitumumab | Folinic acid, fluorouracil, and irinotecan | 4 | PPE, trichomegaly | Complete |
| 3/F/70s | Colon sigma ADK, stage IV | Fingernail (2 PG) | Panitumumab | Folinic acid, fluorouracil, and irinotecan | 2 | PPE, trichomegaly, hirsutism, seborrheic dermatitis | Complete |
| 4/M/50s | Rectum ADK, stage IV | Toenail (2 PR) | Panitumumab | Folinic acid, fluorouracil, and oxaliplatin | 4 | PPE | Partial |
| 5/M/70s | Tonsillar SCC, stage IV | Both (2 PG and 2 PR) | Cetuximab | NA | 1 | PPE | Complete |
| 6/F/70s | Sigma ADK, stage IV | Fingernail (3 PG) | Panitumumab | Folinic acid, fluorouracil, and oxaliplatin | 12 | PPE, severe xerosis cutis, palmoplantar fissures, seborrheic dermatitis | Complete |
| 7/F/70s | Lung ADK, stage IV | Fingernail (2 PG) | Erlotinib | NA | 1 | Acute pancreatitis, palmoplantar fissures | Complete |
| 8/F/70s | Colon ADK, stage IV | Toenail (2 PG) | Capecitabine | NA | 5 | Hand-foot syndrome | Complete |
| 9/M/50s | Colon ADK, stage IV | Toenail (2 PG) | Cetuximab | Folinic acid, fluorouracil, and oxaliplatin | 3 | PPE | Complete |
| 10/F/60s | Tonsillar SCC, stage IV | Fingernail (5 PR and 1 PG) | Cetuximab | Paclitaxel | 2 | PPE, mucositis, onycholysis | Complete |
Abbreviations: ADK, adenocarcinoma; NA, not applicable; PG, pyogenic granuloma; PPE, papulopustular eruption; PR, paronychia; SCC, squamous cell carcinoma.
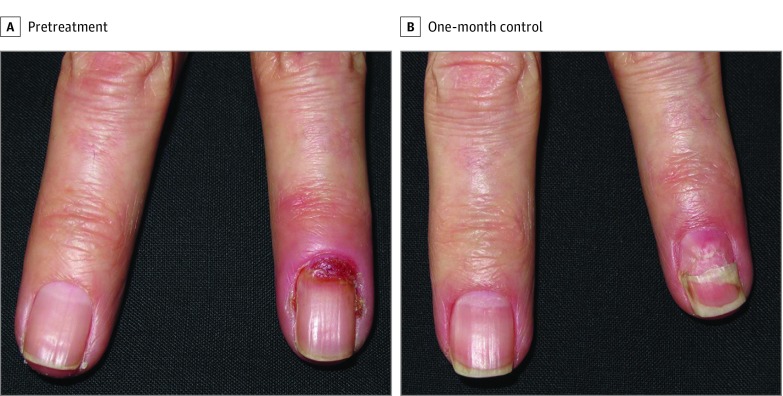
All patients clinically improved with timolol treatment at the 1-month evaluation: complete response in 9 patients (Figure) and partial response in 1 patient, who was receiving treatment with panitumumab for 2 toenails with pseudo-PG. Regardless of the degree of response, all patients reported a high degree of satisfaction and tolerance to treatment. No adverse events were reported within 1-month to 8-month follow-up.
Figure. Fingernail Pseudopyogenic Granuloma.
Note that the secondary onychomadesis is attributable to the initial nail matrix affectation.
Discussion
Epidermal growth factor receptor inhibitors are increasingly used for the treatment of advanced solid malignant tumors. Paronychia and pseudo-PG occur in 10% to 30% of cases, and their appearance is dose dependent, requiring at least 4 to 8 weeks of continued therapy. Their etiopathogenesis remains unknown. Some authors suggest that initial desquamation and thinning of the periungual epidermis, induced by the antineoplastic drugs, would cause onychocryptosis and periungual inflammation, leading to granulomatous tissue outgrowth. Pain secondary to paronychia seriously impairs self-care and daily activities and thus the quality of life of these patients. Management of this condition is usually difficult, with poor response to multiple treatments (eg, surgery, topical antiseptics, antibiotics, corticosteroids), and dose reduction or withdrawal of the antineoplastic agent may be required.
Topical timolol has emerged as a promising treatment for infantile hemangiomas and idiopathic PG. In a recently published series of 10 patients with periungual PG, topical treatment with propranolol cream, 1%, under occlusion has been reported to cure fingernail lesions regardless of the cause, with no response or even worsening in toenail PG. In our series, timolol, 0.5%, gel under occlusion was associated with good response, regardless of the localization. A potential explanation might be the superior activity, increased penetration, or better excipient of timolol gel compared with propranolol cream.
Our open study provides initial evidence of the efficacy, safety, and tolerance of timolol, 0.5%, gel under occlusion for the treatment of paronychia and pseudo-PG associated with antineoplastic treatment. Future randomized clinical trials are required to confirm our findings and provide information regarding the optimal dose and duration of treatment.
References
- 1.Robert C, Sibaud V, Mateus C, et al. . Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 2015;16(4):e181-e189. [DOI] [PubMed] [Google Scholar]
- 2.Piraccini BM, Alessandrini A, Dika E, Starace M, Patrizi A, Neri I. Topical propranolol 1% cream for pyogenic granulomas of the nail: open-label study in 10 patients. J Eur Acad Dermatol Venereol. 2016;30(5):901-902. [DOI] [PubMed] [Google Scholar]
- 3.Piraccini BM, Bellavista S, Misciali C, Tosti A, de Berker D, Richert B. Periungual and subungual pyogenic granuloma. Br J Dermatol. 2010;163(5):941-953. [DOI] [PubMed] [Google Scholar]
- 4.Califano R, Tariq N, Compton S, et al. . Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75(12):1335-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik M, Murphy R. A pyogenic granuloma treated with topical timolol. Br J Dermatol. 2014;171(6):1537-1538. [DOI] [PubMed] [Google Scholar]
- 6.McMahon P, Oza V, Frieden IJ. Topical timolol for infantile hemangiomas: putting a note of caution in “cautiously optimistic.” Pediatr Dermatol. 2012;29(1):127-130. [DOI] [PubMed] [Google Scholar]