Abstract
Integuments form the boundary between an organism and the environment. The evolution of novel developmental mechanisms in integuments and appendages allows animals to live in diverse ecological environments. Here we focus on amniotes. The major achievement for reptile skin is an adaptation to the land with the formation of a successful barrier. The stratum corneum enables this barrier to prevent water loss from the skin and allowed amphibian/reptile ancestors to go onto the land. Overlapping scales and production of β-keratins provide strong protection. Epidermal invagination led to the formation of avian feather and mammalian hair follicles in the dermis. Both adopted a proximal - distal growth mode that maintains endothermy. Feathers form hierarchical branches which produce the vane that makes flight possible. Recent discoveries of feathered dinosaurs in China inspire new thinking on the origin of feathers. In the laboratory, epithelial - mesenchymal recombinations and molecular mis-expressions were carried out to test the plasticity of epithelial organ formation. We review the work on the transformation of scales into feathers, conversion between barbs and rachis, and the production of “chicken teeth”. In mammals, tilting the balance of the BMP pathway in K14 noggin transgenic mice alters the number, size and phenotypes of different ectodermal organs, making investigators rethink the distinction between morpho-regulation and pathological changes. Models on the evolution of feathers and hairs from reptile integuments are discussed. A hypothetical Evo-Devo space where diverse integument appendages can be placed according to complex phenotypes and novel developmental mechanisms is presented.
Keywords: feather, scale, hair, evolution, development, Mesozoic, skin appendage
1. Introduction
The integument includes the skin and associated structures. The gradual evolution of novel molecular/developmental mechanisms in integuments and their appendages allow animals to live in different ecological environments (Fig. 1A). The first and most basic function of the integument is to set up a boundary between an organism and its environment. Within the boundary, internal homeostasis must be sustained. A basic integument function is protection as can be seen clearly in fish scales. Communication was also an early function that persists, since animals have used the integument as a canvas for message displays. In fish, the scales form a protective layer and the diverse shapes of different fins provide scaffolds for different ways of locomotion and other functions. In amphibians, the need to live in both water and land has driven the formation of complicated glandular systems, turning the skin into chemical factories. When reptiles started to appear on land, the formation of effective barriers in the suprabasal epidermis was an essential evolutionary novelty. Enfolding of the skin led to the formation of reptile scales which are used mainly for defense, but also for locomotion and communication. As animals evolved toward endothermy, heat preserving skin appendages, hair and feathers, evolved from scales and contributed to the formation of the mammalian and avian classes. One key feature shared by both appendages is the formation of follicles, with stem cells well protected in the skin. This produces a proximal-distal growth mode which allows for continuous elongation of the appendages. In feathers, the filaments proceeded to evolve branched structures that initially made temperature preservation more effective. Further elaboration of the branching process led to hierarchal branches, making flight possible. In mammals, the evolution of mammary glands for nurturing babies became a cardinal feature.
Fig. 1.
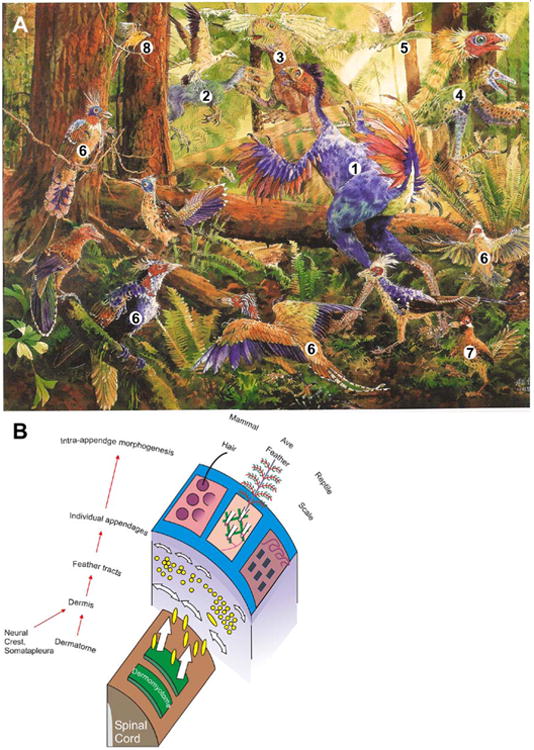
A) Mesozoic creatures and landscape. Life reconstruction of the late Jurassic. Note the diverse integuments and appendages present in the dinosaurs, Mesozoic birds and early mammals.
Reptiles: Caudipteryx (1), Sinosauropteryx (2), Psittacosaurus (3, a beaked dinosaurs); Sinornithosaurus (4), Pterosaurs (5, dinosaurs glide with skin flaps). Birds: Confuciusornis (6), Changchengornis (7), Liaxiornis (8, a small toothed bird). Mammals: Zhangheotherium (9, an early mammal). For 1 - 5, see table 2 and section 3. From Hou et al., 2003. P. 38. Painted by Anderson Yang.
B) Different developmental stages of skin appendage morphogenesis. The principles of skin formation are the same in reptile, birds, and mammals. From dermatomyotomes and other sources, dermal cell precursors migrate in and build presumptive skin and appendages with regional specificities. They share similar hierarchical morphogenesis but acquire variations that lead to different skin appendage phenotypes. Modified from Chuong and Homberger, 2003.
Although vertebrate skin appendages such as scales, feathers, hairs and teeth appear to be very different, they share a number of common developmental pathways, such as the Hedgehog, BMP and Wnt signalling pathways. Variation and innovation in developmental processes are thought to be a key mechanism of organ novelty (Chuong, 1998). The evolutionary origins and diversity of vertebrate integument appendages has long been of great interest (Bereiter-Hahn, 1986). The amazing findings of feathered dinosaurs in China stimulated renewed interest into the evolution of reptilian scales to avian feathers (Sawyer and Knapp, 2003; Prum and Brush, 2002; Chuong et al., 2003). While the integument appendages among reptiles, birds and mammals are diverse, they share common developmental pathways. From the dermomyotome, neural crest, and somatopleura cells give rise to form the dermis. They interact with epithelium to form the skin and skin appendages. During these processes, regional specificities are endowed in development and evolution to generate diverse integuments and their appendages (Fig. 1B). In this paper, we will focus on the amniotes. We will first describe the extant diversity of integuments in reptiles, birds, and mammals. We will then describe the fascinating integument fossils that were recently discovered in Northern China 120-130 million years ago in the Mesozoic time and provide potential missing links of integument appendage evolution (Hou et al., 2003; Zhou et al., 2003). In a cell / molecular biology laboratory, tissue recombination/molecular mis-expression experiments can alter the size, number, and phenotypes of integument organs and provide insight on their development and evolutionary origin (Yu et al., 2002; Plikus et al., 2004). The results of these experiments serve as a basis for discussions of the possible evolutionary relationships and transitional processes that took place during the evolution from reptilian skin to avian feathers and mammalian hairs. A special review issue from J. Expt. Zoology/Molecular and Developmental Evolution Section is dedicated to the topic of Development and Evolution of amniote Integuments (Chuong and Homberger ed. Volume 298B, Aug, 2003). A more detail coverage can be found there.
2. Diversity of integument appendages
The biology of the integument is rich (Bereiter-Hahn, 1986). In this review, we try to choose topics that have implications in the context of Evo-Devo, present new findings with molecular understanding, and highlight future research issues with Evo-Devo implications.
2.1. Diversity of reptile integument appendages
Epidermis
Early reptiles may have appeared during the Carboniferous period about 340 million years ago (mya) (Pough et al, 2001). The reptiles solved the problem of reproduction on land by producing the amniotic egg. Early reptiles probably lived in a hot climate and they evolved a tough, protective scaly integument (Pough et al., 2001). For early amniotes, the adaptation to land from their amphibian ancestor was achieved by a major evolutionary innovation: the formation of the stratum corneum that prevented water loss from the skin and allowed amphibian/reptile ancestors to go onto the land (Maderson, 2003; Alibardi, 2003). The stratum corneum in reptiles is composed of matrix proteins, corneous cell envelope proteins and complex lipids that can prevent water loss from the skin (Alibardi, 2003). Early amniotes then evolved two different strategies to prevent water loss (Maderson, 2003). In Sauropsid amniotes, the ancestors of reptiles and birds, a β-keratinized layer formed above the α-kertinized layer and became the major constituents of scales and feathers. It provided mechanical protection. In Theropsid amniotes, the ancestors of mammals, scales were lost and their α-keratogenic epidermis was strengthened by a mammalian-type HRP (histidine-rich protein).
Scales
The transition from the aquatic to terrestrial environment required more adaptations. The newly evolved epidermis had to provide mechanical protection and prevent desiccation (Landmann, 1986). Reptiles achieved this reinforcement both in the epidermis and the dermis. The cornified area of the epidermis was strengthened by the formation of stiff β-keratin that improved the mechanical resistance of the epidermis and protected the underlying softer, lipid-filled, α-keratin layer (Landmann, 1986; Maderson, 2003). The dermis could be reinforced by dermal ossification (Landmann, 1986). Reptiles solved the problem of flexibility of the exoskeleton by forming scales, through folding the skin with a protruding outer layer and an underlying soft inner layer that became the hinge (Maderson, 1972).
Today there are four orders that represent reptiles: Crocodilia (alligators and crocodiles), Chelonia (turtles and tortoises), Squamata (lizards and snakes), and Rhynchocephalia (tuatara) (Pough et al, 2001). Three typical reptile scale types exist (Maderson, 1965). Overlapping scales is the common type. It has distinct outer and inner surfaces (Fig. 2A). Each overlapping scale has a hinge region providing flexibility between scales. The scale is asymmetric with the hinge region assigned to the posterior end. The outer surface consists of a strongly cornified epidermis, which provides stiffness for the scale. Reduced overlapping scales are found on the heads of squamates, which have a smaller inner surface. Tuberculate scales are found on the body of some lizards, like the Gecko, which has a round surface without an anterior- posterior (A-P) axis (Maderson, 1965). Some lizards, such as the iguana, also have an elongated scale (frill) on the dorsal region of the body (Fig. 2A). Future studies of the growth mode of different types of scale will help us to understand the molecular and cellular bases of scale growth and evolution.
Fig. 2. Examples of integument appendages from reptiles, birds, and mammals.

A) Reptile scales. B) Top, adult chicken foot. H&E stained sections highlighted in the left panel corresponding to the scutate scale and reticulate scale are shown. Bottom, adult chicken body feather. H&E stained sections indicated at the indicated planes corresponding to pennaceous and plumulaceous regions are shown. The dotted lines indicate the ramus. bb, barbule; is, inner surface; os, outer surface; rm, ramus. C) Mammalian skin appendages. Mouse vibrissae hair follicle. H&E staining. Claw morphology: Compared to the long and curved claw in Monodelphis domestica, the claw in the more arboreal species, Marmosa robinsoni, is short. K14-Noggin mutant mice have reduced or no claw compared to wild-type mice (from Plikus et al., 2004 and Hamrick, 2003). Footpads in K14-Noggin and Hoxd13 -/- mutant mice are smaller in size compared to the wild-type mice (from Plikus et al., 2004 and Hamrick, 2003). Volar skin from the digits of Philander opossum and Chironectes minimus (from Hamrick 2003). Dolphin skin. H&E staining.
The development of scales in Squamate reptiles begins with epidermal papillae, which are undulations of the epidermal surface producing symmetric dermo-epidermal elevations (Maderson, 1965; Dhouailly, 1975; 1998). The epidermis becomes undulated to form scale primordia due to differences in growth rate or mechanical forces between the epidermis and dermis (Dhouailly and Maderson, 1984). Four developing stages have been recognized by Alibardi (1996), including the flat bilayered epidermis stage, the symmetric scale anlagen stage, the asymmetric scale anlagen stage and the β-keratinizing asymmetric scale stage. The asymmetric scale anlagen stage in the embryonic bearded dragon (Pogona Vitticepts) is shown in Fig. 2A. None of the placodes (localized elongations of epidermal cells) similar to that of avian feathers have been identified in reptiles (Maderson and Sawyer, 1979). However, it is possible that placode specific molecular markers may be identified in the future, even though there are no evident morphological changes in the epithelial cell shapes of reptile skin.
Other integument appendages
Claws
Some amphibians have claws and some not. Most reptiles have claws. Claws probably start as local epidermal thickenings with special keratinization. In the distal ends of digits, proximal-distal axis can be developed by having a localized growth zone that generates special epidermal cells continuously (claw matrix). Claws can help reptiles adapt to the new terrestrial lives. In some dinosaurs, claws have evolved into weapons and the claw of a tyrannosaur can reach a length of one foot with knife-sharp edges. The molecular basis of claw development has not been addressed yet.
Skin modifications
Some reptiles have developed fin like skin appendages along the mid-dorsal lines. Some have developed skin folds near the neck regions that can be used for communication. Some reptiles have the amazing ability to climb walls. In Geckos, seta developed in the ventral digits, allowing geckos to climb up smooth surfaces and even in upside down positions. This is based on numerous setae whose dimensions are smaller than the diameter of human hairs. Through special retraction motions, they can adhere and de-adhere from smooth surfaces with ease (Autumn et al., 2000).
Molting
The process of epidermal renewal allows for somatic growth, repair, and prevention of cutaneous water loss (Alibardi and Maderson., 2003). Desquamation in mammals, birds, crocodilians, and turtles involves the displacement of single cells from the stratum germinativum to the surface were they are exfoliated individually or in small patches (Landmann, 1986; Maderson et al., 1998). However, a unique phenomenon is associated with lepidosaurian reptiles (e.g. Sphenodon, lizards, and snakes). This involves the synchronized cyclic formation of a new epidermal generation (EG) throughout the entire body during the process of shedding (Maderson et al., 1998). Proliferating cells, originating in the stratum germinativum, move upwards differentiating to form a new inner epidermal generation (IG) located between the stratum germinativum and the intact older outer epidermal generation (OG) (Maderson et al., 1998). Each generation contains up to six different histologically distinct layers. These layers include the oberhautchen, β-layer, mesos, α-layer, lacunar, and clear layer (Maderson et al., 1998). This new IG is histologically similar to the older OG. The interdigitation and subsequent separation of the clear layer from the OG and the subjacent oberhautchen layer of the IG allows the disassociation of the EGs (Alibardi and Maderson., 2003). The subsequent loss of the OG is shed in its entirety or in large pieces. Future study of the molecular basis of scale shedding could illustrate the mechanism of this unique skin regeneration process.
Keratinization
Keratins are distributed throughout the entire scale surface and hinge region in reptile scales. Reptiles have both α and β-keratins. α-keratin molecules show a helical arrangement and form polymers. They exist in the epidermis of all vertebrates and have a molecular weight of about 40-70 kDa. They are well conserved as shown in an example of keratin K12. An epitope recognized by a monoclonal antibody against chicken K12 cross reacts with a similarly sized protein present in a number of vertebrates - from trout to human (Chaloin-Dufau et al., 1993).
To make hard integument appendages (claw, hair, feather, etc.), there were two strategies taken by the amniotes. One is via modifications of α-keratin and associated proteins (see below, under mammals). In Sauropsides, it is by the evolutionary novelty of β-keratin molecules (Gregg and Rogers, 1986; Fraser and Parry, 1996; Alibardi, 2003) which are present only in reptiles and birds. β-keratins have no molecular homology with α-keratins. They have a small molecular weight of about 10-25 kD and exhibit unique arrangements of pleated sheets (Shames et al., 1989; Presland et al., 1989a, b).
In the overlapping scales of squamata (lizards and snakes) and Rhynchocephalia (tuatara), β-keratins are found in the cornified epidermis in the outer scale surface and the hinge region (Baden and Maderson, 1970; Alibardi and Sawyer, 2002), whereas the α-keratins are found in a layer in the lower cornified epidermis throughout the scale (Baden and Maderson, 1970). This distribution of keratin types allows a complete epidermal generation to form before the old cornified layers of the epidermis are shed (Baden and Maderson, 1970). The distribution of α and β-keratin in alligator scale showed a similar pattern as seen in lizards and snakes (Alibardi and Thompson, 2002).
Integument appendages, in a broad sense
These are not traditionally considered skin appendages. However, they are derivatives of integuments, follow the logic of integument appendages, and are best understood as integument appendages.
Teeth
A long held view of the origin of teeth, based on structural and developmental similarities of fish dermal armor and mammalian teeth, is that teeth evolved from dermal armor by internalization of dentin-containing dermal armor into the oral cavity. Although this hypothesis is still controversial, recent work showed that the Eda pathway, homologous to the TNF pathway, is already required for fish scale formation (Kondo et al., 2001), and essential for the formation primary hairs and tooth development (reviewed in Sharpe, 2001). Many reptiles are homodonts, although there are some variations in the size of teeth in different parts of the mouth. In most reptiles, teeth are of a simple conical type. Somewhat flattened teeth are found in some lizards and crocodilians. Turtles have lost their teeth but evolved a horny bill. However, in fossils, there were greater diversities in the shapes of reptilian teeth.
Carapace
The turtle shell is a bony structure which include spine, ribs, dermis and an outer β-keratinized epidermal layer (Loredo et al., 2001). The shell includes a dorsal carapace and a ventral plastron. The growth of the carapace is mediated by the carapacial ridge that is analogous to the apical ectodermal ridge of the limb. The carapacial ridge expresses Msx and FGF10, (Vincent et al., 2003). These works suggest that common mechanisms participate in the early development of the limb bud and a carapace ridge.
2.2. Diversity of avian integument appendages
Birds started to evolve from reptiles nearly 200 mya (Chiappe, 1995; Feduccia, 1999). Birds have one of the most complex forms and physical structures that allow them to live in different ecological environments, including the water, land and sky (Gill, 1994; Lucas and Stettenheim. 1972). Compared with reptiles, the avian integument shows more diversity. Feathers are the most complex vertebrate skin appendages (Lucas and Stettenheim. 1972) and function in insulation, communication, and flight (Chatterjee, 1997; Chiappe, 1995; Feduccia, 1999). Scales are found on the avian foot (Lucas and Stettenheim. 1972).
Scale
Chickens have three major types of scales on the leg; scutate, scutella and reticulate scales (Dhouailly, 1984; Sawyer et al., 1986). The reticulate scales which are on the foot pad are radially symmetric (Fig. 2B). The structure of scutate and scutella scales are similar, although scutella scales are smaller and have a reversed orientation. Both show anterior-posterior polarity. Avian scutate scales and reptile overlapping scales appear similar. Both have the outer surface, inner surface and hinged region (Fig. 2A, B). However, unlike the reptile overlapping scales, avian scutate scales do form placodes (Sawyer, 1972). Five developmental stages of avian scutate scales were described by Sawyer (1972): the preplacode, placode, asymmetrical placode, hump and definitive scale ridge stage. Unlike in feather development, the dermal condensations appear but are difficult to see beneath the placodes of scutate scales (Sawyer, 1972). Similar to reptile scales, the outer surface of avian scutate scales is composed of both β-keratin and α-keratin. The β-keratins are restricted to the stratum intermedium and stratum corneum of the outer scale surface. α-keratins are found in the stratum basale and stratum intermedium of the outer scale surface and throughout the epidermis of the inner scale surface and hinge region (O'Guin and Sawyer, 1982).
Avian reticulate scales do not form apparent placode morphology. Three developing stages have been described by Sawyer and Craig (1977): the prereticulum, reticulum primordia and symmetrical prominent elevation stage. Reptile overlapping scale development goes through similar developmental stages (Maderson, 1965; Maderson and Sawyer, 1979) before they become asymmetric. At the primordial stages, avian reticulate scales do not form placodes and are more similar to reptilian overlapping scales than to avian scutate scales (Sawyer et al, 1986). Regions of the dermis extend to the thick epidermis of the radially symmetric reticulate scale on the plantar (Sawyer and Craig, 1977) (Fig. 2B). The epidermis in avian reticulate scales only expresses α-keratin in the stratum corneum and stratum intermedium (O'Guin and Sawyer, 1982). No β-keratin has been detected there.
Morphologically avian reticulate and scutate scales are similar to reptile tuberculate and overlapping scales. Whether these avian scales are homologous to the reptile scales or are secondary derived structures of birds remains to be decided. The discovery of the four winged dinosaur, Microraptor gui (Xu et al., 2003; see section 3) raises the question on whether the flight feathers on the leg represent a prototype or special adaptation. If it turns out that a winged leg is a prototype in the early dino-bird transition, it would support the notion that avian foot scales are secondarily derived.
Feathers
Feathers on the bird body show hierarchical branch patterns. The major types of avian feathers include contour feathers, remiges, rectrices, downy feathers, etc. (Lucas and Stettenheim. 1972). A typical avian feather consists of a shaft (rachis) and barbs. The barbs are composed of a shaft (ramus) and many smaller branches (barbules) (Lucas and Stettenheim. 1972). Different feathers show variations in symmetry. Down feathers are radially-symmetric. Their rachis is absent or very short. Contour feathers have bilateral symmetry. Flight feathers are bilaterally asymmetric (Lucas and Stettenheim. 1972). A contour feather has a distal pennaceous region and a proximal plumulaceous region (Fig. 2B), so the feather can help the integument function for contour/communication display with the distal portion, but keep warmth with its proximal plumulaceous portion. The pennaceous regions are made of groove shaped proximal barbules and distal barbules that form hooks. Therefore the distal barbules of a barb interlock with the proximal barbules of the barb above, forming a feather vane in a Velcro like mechanism. Plumulaceous regions are made of similarly shaped, elongated barbules. They are fluffy and soft. Barbules on the barbs can be bilaterally symmetric (across the ramus) and slender. The difference in barb configurations is shown in cross sections of pennaceous and plumulaceous feather regions (Fig. 2B).
During avian embryonic development, feather formation starts with a placode, which is composed of elongated epithelia accompanied with dermal condensations (Sengel, 1958). These feather primordia elongate and protrude out to form feather buds. Feather buds are originally radially symmetric, but soon acquire anterior-posterior polarity through interactions with the epithelium. Feathers then start to elongate and develop a proximal-distal axis (Fig. 4). Feathers form follicles which offer advantages over skin appendages that do not, such as scales. The follicle protects the epithelial stem cells and dermal papillae. Localization of the stem cells within a protected environment enables regeneration through feather molting cycles or plucking (Lucas and Stettenheim. 1972). New cell proliferation at the follicle base pushes the more differentiated portions of the feather to the distal end. The follicle also provides mechanical structures for muscle attachment and coordinated movement. For more on feather follicles, please refer to Yu et al., in this issue.
Fig. 4. An example of molecular morphogenesis of integument appendages.

Upper panels show different stages of feather placode, bud, and follicle formation. Major molecular pathways and morphogenetic events are highlighted in the box. Lower panels show cross sections of a feather filament and different stages of branching morphogenesis
Feather filaments go through epithelial invaginations and evaginations to form the barb ridges, which precede the formation of the barbs and barbules. The barb ridges further differentiate into the barb plates, axial plates and marginal plates. Barb plate cells will be keratinized and become barbs, while marginal plate and axial plate cells undergo apoptosis, die and become spaces (Fig. 4; Chang et al., 2004b). The central pulp also undergoes apoptosis allowing the feathers to unfold and assume their characteristic shapes. The barbules on the barbs differentiate to form different shapes adding to barb complexity (Lucas and Stettenheim. 1972). Thus, the branching morphogenesis of feathers is formed. We would like to call this way of branch formation “reverse branching morphogenesis”, in contrast to the “branching morphogenesis” in lung and mammary gland formation. In the later case, branching patterns are generated from differential proliferation of growing bud tips.
Thus feathers are built in hierarchical order (Prum and Dyck, 2003). In each successive stage, they use signaling molecules in different ways (e.g., wnt in Chang et al., 2004a). These molecular pathways have recently been reviewed (Widelitz et al., 2003) and are summarized with morphogenetic events in Fig. 4. The multi-layered morphogenesis modules in feather formation provide the basis for many feather variants selected by fancy bird breeders (Bartels, 2003). Finally, even with skin appendages constructed in the right morphological form, they have to be connected with other systems to be integrated with the organism. For example, accompanying the complex evolution of right feather forms, new muscle connections and neural networks have to be evolved and established before birds can take the flight (Homberger and de Silva, 2003).
Other integument appendages
Claw
Avian claws are used in grasping, climbing and fighting. Most Mesozoic birds have claws in their wings (Hou et al., 2003). Most modern birds lost the claws on their wings. However, newly-hatched hoatzins (Opisthocomus hoatzin) in South America still have a claw on the wing to help them scramble around the treetops (Feduccia, 1999). This wing claw is eventually lost in adult hoatzins.
In chickens, foot claws develop with dorsal-ventral asymmetry at E10 and start to express beta keratin around E11. Claw keratin was cloned (Whitebread et al., 1991). Using antibody staining, epitopes on chicken claw keratins were found to be shared by epitopes on the keratins in cornified beaks and egg teeth (Shames et al., 1991).
The curvatures of the claw have been used as indicators for animal habitats. Flat claws suggest ground dwelling while curved claws imply arboreal habitats. Archaeopteryx possesses curved claws and was likely to be arboreal (Feduccia, 1999).
Turkey beard
In turkey beards, a specialized bristle exists. It does not form a follicular structure, but grows continuously to form finger-like outgrowths. It is hollow, and can be considered cylindrical. It forms simple branches, but does not form the hierarchical levels of rachis/barbs/barbules seen in typical feathers. However, it expresses feather type beta keratins. Is it a feather? This filamentous integument appendage may be considered to be one of the protofeathers (Sawyer et al., 2003b, also see Section 3 for the definition of true feathers).
Combs and wattles
These are wrinkled skin folds located at the top of the chicken head or neck, and are often brightly colored. Their growths are sex hormone dependent. In some bird variants, instead of growing combs, a group (tract) of contour or flight feathers forms on the head.
Molting
Feathers go through molting cycles (Lucas and Stettenheim. 1972; Yu et al., this issue) consisting of a growth phase and resting phase. The growth phase can be characterized by the red pulp (blood vessels) visible in the growing feather shaft. The longer the growth phases, the longer the feathers. The resting phase is represented by the stop of growth, degeneration of pulp, and maturation and fully opening of feather vanes. However, feathers remain attached to the follicles through their shafts. Eventually, differentiation leads these feathers to slough off.
Birds commonly molt twice a year: once in the spring for more attractive plumages, and once in the fall for the more protective plumages. However, the process is highly modulated by the environment: seasons, temperature, nutrition, etc. and the effects are probably mediated by hormones. From the same follicle, the generated feathers do not always have the same morphology, color and size. This is particularly obvious in that flight feathers are preceded by down feathers in the same follicles, and sex hormones transform ordinary brown feathers into spectacularly colorful peacock tail feathers in mature males. Thus every molting event gives the bird a new opportunity to remodel its regenerating feathers, thus allowing birds to alter their integument appendage phenotypes in response to the changing environment. This is an important research issue (Chuong and Homberger, 2003) and the feather is a good model in which to study it, given its continual and physiological regenerative processes.
Keratinization
The skin appendages of reptiles and birds are characterized by the presence of both α and β-keratins (Sawyer et al., 2000). Avian β-keratins are the products of a large family of homologous genes. β-keratin in avian scales and feathers showed strong homologies in the protein coding region (Gregg et al., 1984), which suggested that the feather keratin genes may have evolved from scale keratin genes by a single deletion event (Gregg et al., 1984).
Like the reptilian scales and avian scales, avian feathers have both β and α-keratins. β-keratin was detected in the feather sheath and barb ridge in feather filaments (Haake et al., 1984; Yu et al., 2002; Chondankar et al., 2003). α-keratin has been reported in the feather sheath and barb ridges of developing feather follicles (Chondankar et al., 2003). An antibody to an avian scale β-keratin cross reacts with reptile scales (Sawyer et al., 1986; Alibardi and Sawyer, 2002). These results suggest that common types of β-keratins are present in both avian and reptilian scales. Feathers had evolved their own specific type of β-keratin. Recently, feather-type β-keratin has been found to be expressed in the subperiderm cells of embryonic scutate scales which suggested that the epidermal populations of the scales and feathers of avian embryos are homologous with those forming the embryonic epidermis of alligators (Sawyer et al., 2003a).
Efforts have been made to apply modern immunological methods to further understanding in the origin of feathers. Using antibodies raised against chicken β-keratin, Schweitzer et al., (1999) reported immunological cross reactivity with feather-like structures of the alvarezsaurid dinosaur, Shuvuuia deserti. Together with mass spectrometric data, they suggested that there are β-keratins, similar to that of birds today, in these dinosaurs. The work is original and this possibility is exciting. As the conclusion is critical, much more rigorous experiments will be required to establish it. It would be worthwhile to make biological specimens go through simulated fossilization processes (as much as one can in high pressure and temperature), and learn how to retrieve molecular and immunological properties of these “simulated fossils”. This type of molecular approach, once established, would be revolutionary to link paleontology research with molecular research.
Integument appendages, in a broad sense
Teeth
Mesozoic birds like Archaeopteryx have teeth and the phylogenetic derivation of modern birds indicates that the absence of dentition was a secondary event, occurring approximately 60 million or more years ago (Huysseune and Sire, 1998). During evolution, they gradually lost teeth as the beak evolved. We recently reported a Mesozoic wading bird, Longirostravis, which has several teeth left in the tip of the bill (Hou et al., 2004) (Fig. 3A-C). The attempt to regrow chicken teeth is described in section 4.3.
Fig. 3. An example of a Mesozoic bird to show the intermediate integument phenotypes.
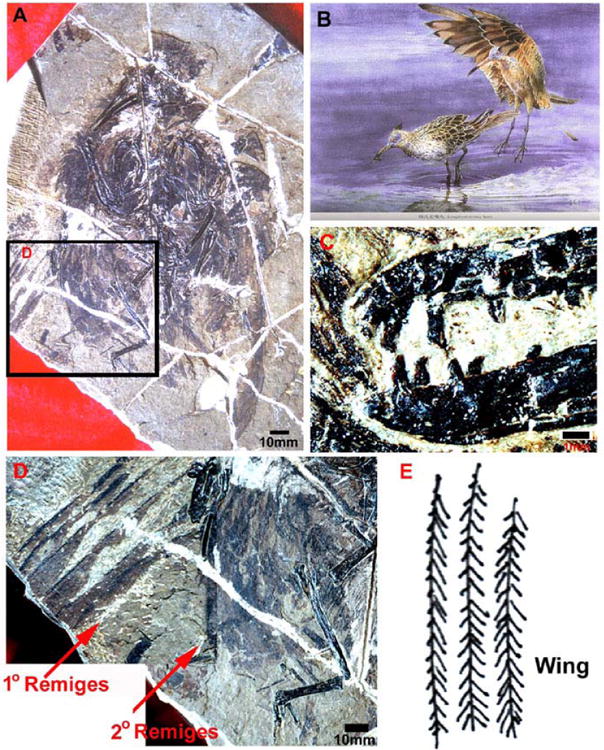
Evolving creatures at this time have overlapping integument phenotypes such as feathered dinosaurs (Fig. 1A, Table 1) or toothed birds. This Longirostravis is the earliest bird we know that has a probing trophism. A) A fossil of the Longirostravis unearthed in the Jehol Biota from the Yixian Formation in northeastern China. B) An artist's conception of the appearance of Longirostravis in life (from Fossil Birds of China, Hou et al., 2003). C) A close up view of the feeding apparatus, showing the presence of teeth within the beak. It is likely the earliest bird to live in a wading habitat. From Hou et al., 2004. D, E) A close up view of the primary and secondary remiges (flight feathers) and their tracings. Note the feather vanes are long and narrow and already start to show left-right asymmetry.
Beak
Beaks are the formation of hardened horny sheaths on the snout. Beak-like structures also existed in some ancient dinosaurs (e.g., Psittacosaurus, Fig. 1A) as well as in current turtles. It is possible that beak-like structures may have evolved independently more than once. In birds, the beak has become a unique feeding apparatus since Mesozoic time (Hou et al., 2003; Fig. 1A, 3). The diverse shapes of the beak are classical examples of evolution (Darwin, 1859; Grant, 1986). Morphogenesis of the beak consists of three major components: the outgrowth of beak primordial mesenchyme (skeleton), the integument inside the oral cavity (oral mucosa, teeth) and the integument covering the snout (horny sheath). The horny sheath exhibits a thick layer of special β-keratin. In the chicken, it starts to form in the distal beak primordia around embryonic day 10. An egg tooth forms at the upper surface of the distal upper beak. It is a special keratinized structure, not an enamel containing type of tooth. It is used for the newborn chick to open the egg shell.
2.3. Diversity of mammalian integument appendages
Due to a lack of fossil evidence, evolution of the mammalian integument remains largely unknown. From the Mid-Permian to Early-Triassic about 200 mya, the early therapsid reptiles may have evolved an integument capable of limiting water-loss and protection from the colder environment (Ruben and Jones, 2000). At that time, some sensory hairs, vibrissae and maybe pelage may have formed (Maderson, 1972; 2003). Some speculations are made in section 5, and here we will examine current mammalian integument appendages.
Hairs
Hair is the major integument appendage of mammals. The driving force to form hairs is likely to be thermoregulation. Hairs can also be distributed with regional specificity for different functions such as communications, protection from direct sunlight, sensory perception, camouflage or sexual attraction. For instance, a mane grows around the neck of lions and on the dorsal region of a horse's neck. There are multiple types of hairs, such as pelage or vibrissae (Sundberg, 1994). There are two major types of pelage hairs: guard or primary hairs and secondary hairs. Auchene, zigzag and awl are three different types of secondary hairs (Nakamura et al., 2001). In many instances, secondary hairs form an underfur and serve to insulate the animal. Vibrissae, which are found at the facial region and commonly referred to as whiskers, are very long and stiff. They serve to sense the animal's immediate environment (Waite and Li, 1993). The follicle structure of vibrissae is different from pelage hairs. The vibrissa follicle is surrounded by large blood sinuses enclosed in a thick collagen capsule (Fig. 2C). Vibrissae are vastly innervated by the sensory nerve endings of trigeminal nerves (Oliver, 1967).
Hair follicles arise as a result of complex morphogenetic interactions between the epidermis and mesenchyme (Hardy, 1992). Hair follicle development is conventionally divided into induction, morphogenesis and differentiation stages (Wu and Chuong, 2000). Upon induction the epidermal placode appears first as a thickening of the flat epidermis. Aggregation of the mesenchymal cells is seen underneath the placode. Later during the induction stage, the epidermal placode grows downwards and forms the hair germ. During the morphogenesis stage, mesenchymal aggregates condense into distinct dermal papillae and the hair germ epithelia reorganize to wrap around the dermal papilla resulting in a hair peg. The bottom portion of the hair peg transforms into the hair matrix that starts to form the inner root sheath, while the peripheral portion of the base and the upper portion of the hair peg become the outer root sheath (Fig. 2C). Next, during the differentiation stage, proliferation in the hair matrix continues and the first hair fiber forms in addition to the inner root sheath. The hair bulge appears as a distinct prominence in the upper portion of the outer root sheath. This region harbors stem cells (Cotsarelis et al., 1990). Above the bulge, a small population of outer root sheath cells gives rise to sebocytes that grow into a sebaceous gland (Yang et al., 1993). The ratio of TCF3 and Lef 1 may regulate the fate of bulge stem cells to become either hair, sebaceous glands, or skin epidermis (Merrill et al., 2001). As the hair fiber continues to form, it reaches the skin surface through a hair canal that allows the hair fiber to grow out from the follicle.
The hair follicle is comprised from epithelial and mesenchymal components (Lane et al., 1991). The outer root sheath (ORS) is continuous with the epidermis at the skin surface and extends downwards all the way to the hair follicle bulb. The hair bulge harbors hair follicle stem cells and is located in the upper part of the ORS. Hair fibers and the inner root sheath (IRS) are produced in the epithelial matrix at the very bottom of the hair follicle. A medulla, cortex and cuticle can be distinguished in the hair fiber. The dermal papilla (DP) and dermal sheath (DS) constitute mesenchymal components of the hair follicle. The dermal papilla is located at the bottom of the hair follicle and is surrounded by an epithelial matrix. The DP is believed to control hair formation (Jahoda et al., 1984) by regulating epithelial cell proliferation and differentiation (Matsuzaki and Yoshizato, 1998). The dermal sheath surrounds the hair follicle from the outside and is confluent with the dermal papilla at the bottom.
Based on the changes of transgenic mice and knock out mice, the involvement of many molecular signaling pathways has recently been identified. These pathways include Wnt, beta catenin, Eda, Shh, BMP, FGF, Notch, etc. They have recently reviewed (e.g., Botckarev and Paus, 2003) and will not be elaborated here. In principle, we can appreciate that the pathways are shared by different ectodermal organs (Chuong, 1998) and examples of noggin/BMP and Eda are discussed.
Other integument appendages
Horns and other variations of hairs
Horns are specially keratinized structures and usually serve as a weapon for defense or attack. The horn of a rhinoceros is made of multiple hardened coalesced hair shafts (Lynch et al., 1973). New horn epidermal cells are inserted at the base. Numerous modified hair follicles initially form a cluster and are gradually arranged in a circle to give the horn a tube-like configuration.
In some whales, the vibrissae hair has been modified to detect water vibrations caused by prey (Balcomb, 1984; Leatherwood and Reeves, 1983; Winn and Winn, 1985). These hair follicles aid the whale to locate prey in close proximity.
Hair can be modified to form different skin appendage structures. For example, in the armadillo, the hair in the back has been compacted and hardened into a large scale-like structure (Patterson, 1978). This hair-scale serves to protect the animal from the environment and predators.
Claw and hoof
Claws and hooves are keratinized appendages on the tips of mammalian digits. Nail development has been described for humans and claw development has been described for cats and rodents (Hamrick, 2003). The development of nails or claws begins with an epithelial thickening (placode), which is the first sign of induction on the dorsal surface of each digit (Chapman, 1986). A proximal claw fold develops as the epidermal thickening invaginates and later forms part of the germinal claw matrix. Cells of the germinal matrix then differentiate to produce a keratinized layer over the nail/claw bed.
Some terrestrial mammals evolve hooves. A hoof is a thick keratinized layer that wraps around the distal limb. The dermal - epidermal junction of the hoof develops a series of invaginations (papillary body) that may provide mechanical properties required for the hoof (Bragulla, 2003). The hoof can be considered an exaggerated exhibition of claw/nail morphogenesis.
Sweat gland
Sweat glands develop via invagination of epidermal cells. Eccrine sweat glands develop as the down growth of the epidermis into the dermis. They start as a budding of the basal layer of the epidermis. The bud further grows downward in the form of solid cylinder. Then its proximal part coils to form the secretory body, while the distal part develops lumen. Apocrine glands originate closely to the hair follicles, so that their ducts open into the hair canals above the sebaceous glands (Moore KL, 1998).
Ectodermal dysplasia in human and mouse is a group of genetic diseases that exhibits multiple ectodermal organ abnormalities based on a single genetic defect (Grüneberg, 1971; Mikkola and Thesleff, 2003). This suggests these ectodermal organs, hairs, nails, sweat glands, salivary gland, etc. share signaling molecular pathways. Among them, the ectodysplasin (Eda) pathway plays an important role. Mice with defects in different components of the Eda pathway, such as Eda (ligand) and Edar (receptor) fail to develop sweat glands. Humans with hypohidrotic ectodermal dysplasia syndromes have a defective Eda pathway, and form similarly abnormal sweat glands (Monreal et.al., 1999). Recently we showed that the BMP pathway regulates sweat gland morphogenesis (Plikus M, 2004). When noggin, a BMP antagonist, is overexpressed in the basal layer of the skin, sweat glands in the footpad are transformed into hair follicles. By blocking BMPs, Noggin may abort sweat gland induction and induce hair follicles instead, or may trans-differentiate the fate of induced sweat gland primordia into hairs.
Footpad
Some terrestrial mammals evolve footpads. They are characterized by thickened dermis. During development, BMP4 is expressed in the mesenchyme where footpads will form. There is more cell proliferation in the dermis of developing footpads. In adults, BMP2 is expressed in the footpad epidermis. Suppression of BMP mediated interactions in K14 noggin mice showed reduced footpads (Plikus et al., 2004; Fig. 2C). Hox d13 mice show reduced footpad formation (Fig. 2C, from Hamrick, 2003). Hox d13, BMPs and noggin may function along the same pathway for the formation of footpads. They may also be used to “morpho-regulate” (see section 4.4) the size of the footpads for adaptation to different niches.
Papillary ridges and variations
Mammalian digit skin exhibits various morphogenetic features that improve the function of fingers and toes. Using arboreal, terrestrial and aquatic environments, Hamrick (2003) compared the distal limb integument structures of opossums. The terrestrial Monodelphis exhibit long, curved claws, while the arboreal Marmosa show small claws (Fig. 2C) but a large volar pad with well developed papillary ridges to aid its tree climbing. In the feet of the water opossum, Chironectes, epidermal scales replaced papillary ridges. Around each scale, there are finger-like cones that may serve tactile functions under the water.
Dolphin skin
Dolphins lost their hair. However, an extraordinary form of papillary ridge forms on the trunk of Dolphins. It is surmised that the function of these ridges serves to produce laminar flow (Carpenter et al., 2000). Laminar flow reduces the amount of drag on the dolphin as it moves through the water environment. Thus the energy output required from the dolphin can be most efficient. However, hydrodynamic drag still exerts extreme forces on the skin and requires extra support to prevent denuding. The dermal papillary ridges exaggerated in the dolphin skin tissue may provide this support (Fig. 2C). The deeply inserted ridge may also help transmit mechanical stimuli.
Cycling
Hairs go through cycling: anagen during which hairs grow, catagen during which hairs are destroyed, and telogen during which hairs rest. Exogen is when the club hairs fall off, which otherwise can remain attached to the old follicles. Many molecular pathways that can accelerate or arrest hair cycles have been reported. However, the clock that drives the hair cycle remains unknown. These are recently reviewed and will not be elaborated here (Botckarev and Paus, 2003). We will just mention some interesting aspects that relate hair cycles to the environment. Season is one major factor. In some horses, one layer of hair is there all year around, while another group of thick hair follicles will grow only if the animal is exposed to very low temperatures during the cold winter months in some climates. If horses are kept indoors, these “winter coats” will not grow (comments by owners of horse ranch). This suggests that adjacent hair follicles can be under different kinds of hair cycle control. Some animals form compound hair follicles (more than one hair from one hair follicle, also see section 4.4 and Fig. 5B) in the winter, but simple hair follicles (one hair per follicle) in the summer. Snowshoe hares have brown fur in the summer but change to white fur in the winter, indicating different melanocyte behavior in hair follicles. As the mammalian integument is critical for temperature control and message display, it is understandable that animals use hair shedding and regeneration as an opportunity to renew the types of integument appendages that will serve them best at the time. It will be most interesting now to learn how these environmental factors are linked to the hair cycles at the molecular level. Some of these effects may be mediated by prolactin, but much remain to be studied (Johnston and Rose, 1999).
Fig. 5. Morpho-regulation of integument appendages.
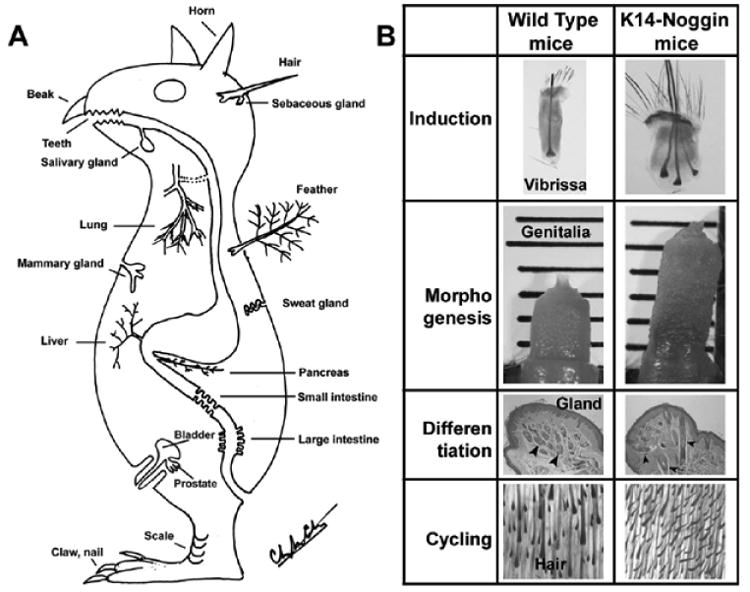
An example is shown in which multiple ectodermal organs are affected when the BMP pathway is perturbed using K14 driven expression of noggin. A) A prototypic animal showing different kinds of epithelial appendages (from Chuong, 1998). B) Changes of ectodermal organs in K14 noggin mice (from Plikus et al., 2004).
Hair is an organ with robust regeneration ability. If plucked during injury, hairs can regenerate as long as the dermal papillae remain. The hair follicle is the main reservoir of stem cells or stem cell like cells (Rochat et al., 1994; Taylor et al., 2000; Ferraris et al., 2000). Recent molecular understanding has made hair follicles an excellent model for stem cell research. They may not only form hairs, but also serve as sources for other organs such as the hematopoietic system (Lako et al., 2002).
Keratinization
α-keratins are the main structural proteins of the epidermis (Fuchs, 1995) and are present in mammalian skin and skin appendages. They form acidic and basic pairs. Mammals do not have β-keratin. They either branched out before the evolution of β-keratin in the reptiles or lost β-keratin that existed in their reptilian ancestors (Maderson, 2003). Hard tissues, such as the hair, nail and claw, contain α-keratins with a high percentage of trichohyalin and other associated proteins, particularly high sulfur proteins, to increase their mechanical resistance (Alibardi, 2003; Thibaut et al., 2003). In human hair follicles, hair keratins exhibit distinct expression patterns. For example expression of human Ha1 starts at the transition of the matrix and the cortex and continues throughout the lower and middle portions of the cortex. Ha2 and Hb2 keratins are specifically expressed in the hair cuticle (Langbein et al., 1999; Langbein et al., 2001). Differential expression of these hair keratins and associated proteins in different mammals may confer different textures and qualities for various hair types.
Integument appendages, in a broad sense
Teeth
Mammals are heterodonts. They have teeth with different forms and functions in different parts of the tooth row (Weiss et al., 1998). Modern mammalian dentitions include three or four kinds of teeth. Incisors have a simple conical shape and are responsible for securing food. Canines serve for piercing food and attacking prey with a conical shape and a sharp point. Premolars and molars developed complex crown patterns and serve a chewing function. Specialization of the teeth in mammals allows them to feed on versatile food sources and is an evolutionary benefit. This complex mammalian dentition is distributed along the proximal-distal axis of the jaw and is in part determined by the homeobox-containing gene families such as Dlx, Lhx and Gsc (Cobourne et al., 2003). Variations in the timing and strength of the activity of many morphogenetic pathways are involved in tooth development (BMP, Shh, FGF, WNT) and the homeobox genes result in the formation of teeth with various shapes and sizes. In part these processes are coordinated within the enamel knots - transient structures of the developing teeth (Jernvall et al., 2000). In addition to being morphologically complex, dentition in many mammals shows different growth strategies. Teeth can either stop growing upon the completion of their development, or they can grow continuously throughout their life. In mice for example, incisors grow continuously, while molars do not. However in other mammals molars can grow continuously throughout their lifetime. Vole and rabbit molars are like this. Different fates of the tooth stem cell population lie at the root of these differences.
Mammalian teeth are composed of two structurally and functionally different parts: crown and roots. During development the crown forms first and roots second. The cervical loop regions of the teeth are believed to be the reservoir of the epithelial stem cells and they supply both crown and roots with “building material”. Developmentally, mouse molar cervical loops switch from making crowns to making roots. Upon this switch molars cease their growth. However, in voles cervical loops continue to produce crowns throughout adulthood, resulting in continuous molar growth. Likewise, cervical loops in mice incisors do not degenerate and continuously produce crowns. Different timing in the “crown/root” switch activation can result in a whole array of tooth phenotypes seen in various mammals (Tummers et al., 2003).
Mammary glands
Mammary glands may not sound like typical skin appendages, but they actually are derivatives of the skin. Their induction involves the formation of an epithelial placode and dermal condensation (reviewed in Veltmaat et al., 2003). Several molecular pathways have been shown to be involved in mammogenesis (reviewed in Veltmaat et al., 2003). For instance, they are dependent on Wnt/β-catenin signaling. This is evidenced by K14 DKK mice transgenic and Lef-1 knock out mice, whose Wnt/β-catenin signaling pathways are inhibited. These mice do not form mammary glands, hairs or teeth (Andl et al., 2002; van Genderen et al., 1994). The formation of the mammary gland is critical to feed the young and is the foundation of the mammalian class. Nursing offers close contact between a pup and its mother and offers ample opportunity for training to foster the transfer of knowledge leading to higher intelligence (Peaker, 2002). Growth factors and immune factors in the milk help to protect and mature the developing infant (Goldman, 2002; Oftedal, 2002). Mammary glands are believed to have evolved from ancient apocrine glands associated with hair follicles (Oftedal, 2002). The secretion of nutrient rich milk probably began in therapsids, such as cynodonts. In today's mammals, they form along milk lines which extend from the axilla to the pubic regions (Grossl, 2000; Veltmaat et al., 2003).
Tongue papillae
On the surface of the tongue, papillae form in regular patterns. They exhibit major morphogenesis signaling molecules such as Shh, BMP2, 4, FGF 8, etc. (Jung et al., 1999). Recombination experiments showed that the morphogenesis of fungiform papillae goes through periodic patterning process and involves epithelial mesenchymal interactions (Kim et al., 2003). Thus fungiform papillae can be considered small epithelial appendages. Fungiform papillae can be considered to be small epithelial appendages, which are formed via the epithelium and mesenchyme interactions. Filiform papillae exhibit hair type keratins and association with hair evolution is hypothesized by Dhouailly and Sun, 1989 (section 5.2).
External genitalia
Copulatory organs also result from epithelial-mesenchymal interactions including the skin (Yamada et al., 2003). In a broad sense, they can belong to the category of integument appendages. In the distal end of the growing genital tubercle, there are BMP4. Furthermore, on the surface of the mouse penis, there are numerous periodically arranged BMP4 expressions during development. They then become hair spines. Interestingly, in K14 noggin mice, the size of penis increases while the differentiation of hair spines is inhibited (Plikus et al., 2004; Fig. 5A, B).
3. Fossil records of integument appendages from Mesozoic reptiles and birds
The discoveries of many intermediate forms of feather-like appendages from the Jehol Biota in China brought many new insights in the evolution of feathers (reviewed in Chuong et al., 2001; Sawyer and Knapp, 2003; Chuong et al., 2003). The Jehol Biota spreads across the Northern part of China and contains fossils of various organisms living 120-145 mya. It is a geological layer representing the transition of from mid-Jurassic to early Cretaceous. Because of the geology, many soft integuments of these Mesozoic creatures were well preserved (Chen et al., 1998; Zhou et al., 2003). These include different kinds of reptiles, Mesozoic birds, and mammals (Fig. 1A). Most interesting, there are many “intermediate species” with characteristics of both birds and reptiles that lived in Mesozoic times. They are extinct now, replaced by more efficient reptilian and avian species. However, these fossils provide multiple clues on how integument appendages may have evolved. One of the examples of Mesozoic birds is the newly discovered Longirostravis, the long rostrum bird (Fig. 3, Hou et al., 2004). This 120 million year old fossil has a long and sharp beak with 10 conical shaped teeth in the distal end. It is the earliest bird that starts to show modulations of beak shapes, and, with long legs, represents the earliest known wading bird. Its feathers already have some asymmetry, suggesting that it is a reasonably good flyer.
There are many Mesozoic reptiles that have elongated branched appendages that appear to be precursors of today's feathers (Hou et al., 2003). Many of these feather-like appendage-bearing dinosaurs belong to the group of theropods. They were carnivorous, fast moving bipedal dinosaurs with small forelimbs but long hands consisting of three digits for grasping prey (Sereno, 1999). The following section introduces some of these extra-ordinary creatures (Table 1).
Table 1. Summarize the integuments in the feathered dinosaur.
| Animal | Classification | Characteristics of skin appendage | Shape | Reference | |
|---|---|---|---|---|---|
| Sinosauropteryx |

|
Theropod Coelurosauria | Filaments (Protofeather), no regional specificity |

|
Chen et al., 1998 |
| Beipiaosaurus |

|
Theropod Therizinosaur | Filaments (Protofeather) |

|
Xu et al., 1999a |
| Suvuuia |

|
Alvarezsaurids | Fibers organized in small clumps |

|
Schweitzer et al., 1999 |
| Sinornithosaurus |

|
Theropod Dromaeosaur | Filaments, having two types of branching structure, no barbules: |

|
Xu et al., 1999b; Xu et al., 2001 |
| Microraptor zhaoianus |

|
Theropod Dromaeosaur | Have rachis, true feather? |

|
Xu et al., 2000 |
| Caudipteryx |

|
Theropod Oviraptorosaur | Different feather tracts. Bilateral symmetric feather in wing and tail. True feather? |

|
Ji et al., 1998 |
| Protarcheopteryx |

|
Theropod Maniraptora | Bilateral symmetric feather on wing and tail which have rachis, barbs, barbules, body covered plumulaceous feather. |

|
Ji et al., 1998 |
| Unnamed |

|
Theropod Dromaeosaur | Three types of filamentous structure: single fibres, long ‘sprays’ of fibres and symmetric feather |

|
Ji et al., 2001 |
| Unnamed |

|
Theropod Dromaeosaur | Symmetric feather, have central rachis and symmetric barbs. |

|
Norell et al., 2002 |
| Microraptor gui |

|
Theropod Dromaeosaur | Asymmetric flight feather in both wing and leg. The body was covered by plumulaceous feathers. |

|
Xu et al., 2003 |
| Psittacosaurus |

|
Ceratopsia Psittacosauridae | Long and thick bristle-like, non-branched integumentary structure |

|
Mayr et al., 2002 |
Sinosauropteryx was the first feathered theropod dinosaur found in the Jehol Biota (Chen et al., 1998), which has “fuzz fibers” on the body, especially along the dorsal midline. These filaments are rather homogenous over the body without regional specificity (Table 1, Fig. 1A). The appendages are hollow and appear to have a short shaft with barbs, but lack further branches. They appear to be like down feathers without any aerodynamic properties and were probably used for insulation. These filaments may represent “proto-feathers” or some early branching skin appendages (Chen et al., 1998).
Two theropods, Beipiaosaurus and Sinornithosaurus, had large patches of filament-like integumentary structures preserved on the forelimbs, hindlimbs and body (Xu et al, 1999a, b) (Table 1, Fig. 1A). Some of the filaments seem to have branching distal ends. These primitive filaments appear to be hollow, reflecting the cylindrical feather filament. Further analyses (Xu et al, 2001) showed that skin appendages on Sinornithosaurus have compound structures containing multiple filaments, which are joined together. These branched structures were either similar to the avian downy feathers or similar to avian pennaceous feathers, but lacking barbules to form a closed pennaceous vane.
Modern feather shapes skin appendages were first found in Caudipteryx and protarchaeopteryx (Ji et al., 1998) (Table 1, Fig. 1A). Caudipteryx evolved different types of feathers over different body regions, indicating the establishment of feather tracts as an evolutionary novelty. Specialized functions for each body part could evolve and enrich integument function. Caudipteryx formed spectacular pennaceous feathers in both the wing (remiges) and tail (retrices) with tapering shafts. The bilaterally symmetric pennaceous structures in Caudipteryx and Protarchaeopteryx have been accepted as vaned feathers (Prum and Brush, 2002). However, the vanes lacked the asymmetry required for flight and were probably used for display to either attract or frighten others. Protarchaeopteryx also had bilaterally symmetric pennaceous feathers. The tail rectrice feathers of Protarchaeopteryx were plumulaceous in the proximal part and pennaceous above the mid-shaft region (Ji et al., 1998). The vaned Protarchaeopteryx feathers appeared to be structurally transitional between the proto-feather-like structures of Sinosauropteryx and the modern feathers of Archaeopteryx.
Modern feathers were also detected in other non-avian theropod dinosaurs. Ji et al (2001) reported an unnamed Dromaeosauridae covered with filamentous feather-like structures over its entire body (Table 1). Three types of filamentous structures were identified in this specimen. The first type had single fibers. The second type had long plumulaceous fibers. The third type had symmetric pennaceous feathers, which may have barbules. This type of pennaceous feathers with a rachis and symmetric barbs were also found in a different species of Dromaeosauridae (Norell, et al. 2002) (Table 1).
The smallest known non-avian theropod dinosaur, Microraptor Zhaoianus (Xu et al., 2000), displayed a more advanced filament pattern near the femur. The filaments are long and contain a rachis (Table 1). The fossil suggests that true feather structures may have already existed in these dinosaurs.
The most interesting discovery among the feathered dinosaurs was the four-winged dinosaur recently reported by Xu et al (2003), Microraptor gui of Dromaeosauridae (Table 1). Both fore and hind limbs were covered with pennaceous feathers arranged in a similar pattern. Feathers at the distal limb positions had asymmetric vanes. The remiges were preserved with the primary remiges longer than the secondary remiges. This may be for improved aerodynamics as similar patterns are observed in modern birds. The body was covered by plumulaceous feathers. The “flight feathers” in the hind limb are not well designed for active flight resulting from flapping the wings. The creature may have adopted a gliding behavior in the flourishing Mesozoic jungles, gliding from one tree to another as seen in some modern mammals.
Recently, a bristle-like, non-branched integumentary structure was found in the non-theropod dinosaur (Mayr et al., 2002). They are in the tail of the horned dinosaur (parrot-beaked dinosaur), Psittacosaurus (Table 1, Fig. 1A). These bristle-liked structures are much longer and thicker than the proto-feathers in Sinosauropteryx and Sinornithosaurus, and were interpreted as cylindrical and possibly tubular epidermal structures. They may not be homologous structures as those integument appendages on the Theropods. Cylindrical configuration during the formation of feather filaments is a character considered very important in the first step of feather evolution (Prum and Brush, 2002, Table 2).
Table 2. The similarity and difference of skin appendages among reptile, bird, mammals and fossils.
| Reptile | Bird | Mammal | Reptile with branching skin appendage and feathered dinosaur |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuberculate scale |
Overlapping scale |
Scutate scale |
Reticulate scale |
Feather | Bristle in Turkey beard |
Hair |
Longi- squama |
Psittaco -saurus |
Sinosauro- pteryx |
Sinornitho -saurus |
Micro- Raptor gui |
|
| Placode | ? | ? | + | ? | + | ? | + | ? | ? | ? | ? | ? |
| A-P axis | - | + | + | - | + | ? | + | + | ? | ? | ? | ? |
| P-D axis | - | - | - | - | + | + | + | + | + | + | + | + |
| Follicle | - | - | - | - | + | - | + | - | + | ? | ? | + |
| Mesenchym al core | + | + | + | + | + | + | - | - | ? | ? | ? | ? |
| Cylinder | - | - | - | - | + | + | - | - | +? | +? | +? | ? |
| Simple branching | - | - | - | - | + | + | - | + | - | + | + | + |
| Hierarchical branching | - | - | - | - | + | - | - | - | - | - | + | + |
| L-R asymmetry | - | - | - | - | + | - | - | - | - | - | - | + |
| Regeneration | ? | ? | - | - | + | ? | + | ? | ? | ? | ? | ? |
| α-Keratin | ? | + | + | + | + | ? | + | ? | ? | ? | ? | ? |
| β-Keratin | ? | + | + | - | + | + | - | ? | ? | ? | ? | ? |
-, no; + yes; ? not sure
The Mesozoic landscapes shaped variable skin appendages in non-avian theropod dinosaurs. These skin appendages displayed a spectrum, from non-branched filaments (Sinosauropteryx) to branched filaments (Sinornithosaurus) to symmetric pennaceous vanes (like Caudipteryx) to asymmetric pennaceous vanes (Microraptor gui). Many of these skin appendages are considered to be possible homologues of avian feathers. Mesozoic branched structures are representatives of reptiles/birds in evolution, which fit some criteria of true feathers, such as hierarchical branching (rachis, barb and barbule structures) and opened vanes, even if their information of follicular structures are unavailable (Sawyer and Knapp, 2003, Chuong et al., 2003). However, the discovery of feathers on these non-avian dinosaurs indicates that feather or feather-like skin appendages were evolving before birds and flight (Norell et al., 2002). Some single dissociated feather-like appendages from the same time are also found from Mesozoic birds or dinosaurs (Chuong et al., 2003).
Could we call these feather-like appendages proto-feathers or real feathers? The term, epithelial appendage or skin appendage, is a much broader name that includes all special derivatives of epithelial structures (Chuong, 1998). However, the term feather is limited to those that share similar growth modes, most of the developmental processes, and many of the biochemical properties. Chuong et al (2003) has proposed 5 characteristics of modern feathers. 1). Have localized zones of proliferating cells positioned proximally, with a proximal – distal growth mode. 2). Forms hierarchical levels of branches of rachis, barbs and barbules. Barbs form by differential cell death, and can be bilaterally or radially symmetric. 3). Have a follicle structure, with mesenchymal core wrapped inside during development, forming the pulp. 4). When mature, the two sides of the feather vane face the previous basal and supra-basal layer, respectively. The pulp is gone. 5). Have stem cells and a dermal papilla in the follicle, hence the ability to go through a molting cycle physiologically and to regenerate after plucking. A summary table comparing these different extant and past appendages is shown in Table 2.
4. Laboratory experiments showing the plasticity and regulation of skin appendages
In the laboratory we now can produce analytical and mis-expression experiments that provide insights into the Evo-Devo of integument appendages. In this section, we will first review the classical tissue recombination experiments that laid down our initial understanding of this process. We will then show 3 examples of gene-mis-expression using the chicken model. The first example is about changing the balance between the feather barb and rachis formation. The second example represents the gain of a pathway: growing feathers from scale epidermis. The third example represents the reactivation of a lost pathway: growing teeth from the chicken oral mucosa. Finally, we will use genetic methods to test the activity levels of one major pathway (BMP) in the integument (noggin expression driven by K14) and report the plethora of ectodermal organ phenotypes.
4.1 Classical tissue recombination experiments
Classical experimental embryology experiments showed that the differentiation of skin appendages is the result of epidermal and dermal interactions (Dhouailly, 1975, 1977). The formation of skin appendages starts by having a region of competent epidermis. Through interactions, the mesenchyme determines the location and type of appendages that will form. Hetero-specific experiments pose an interesting question as to what extreme the tissues can be pushed. In a classical set of experiments, Dhouailly did epithelial - mesenchymal recombinations among mouse hairs, chicken feathers, chicken scales and reptile scales (Dhouailly, 1975, 1977). The results showed that the class specificity of skin appendages is epidermis-dependent, while their region-specific architecture (such as size, shape and distribution) is dependent on the dermis. These results indicate that tissue messages can be understood across species but each species can only respond within their genetic capability. When the epithelium is more advanced, they may be semi-committed and, upon induction, have limited potential. On the other hand, when the mesenchyme is faced with pluripotent cells, it can reset and guide these cells to form different types of appendages. For example, basal cells from the central cornea can be induced to form sweat glands when they are confronted with plantar dermis, and induced to form hair follicles and sebaceous glands when they are confronted with upper lip or dorsal dermis (Ferraris et al., 2000).
Retinoid pathway activities can induce epithelial metaplasia (Hardy and Bellow, 1978; Blanchet et al, 1998) and appendage phenotypes. When retinoic acid is added before phenotypes are irreversibly determined, scales are converted to feathers in chickens (Dhouailly et al., 1980), and hair germs are converted to gland-like structures in mouse (Hardy et al., 1990). There are regional differences of the Hox expression patterns in chicken skin which prompted us to suggest that the Skin Hox code may determine the phentoypes of skin appendages (Chuong, 1993). In retinoic acid induced scale - feather metaplasia, the expression of Hox D13 in the scale region disappeared, and became more similar to that of the feather dermis (Kanzler et al, 1997).
Today stem cell and de-differentiation research are active. What used to be called “metaplasia” changes were actually pioneering research on how to engineer stem cells. We should go back to these classical studies and identify their molecular changes.
4.2. Molecular conversion during scale and feather morphogenesis
It has been shown that the expression patterns of different molecular pathways effects their roles in the developmental processes underlying epithelial appendage development. Studies in the recent 10 years by us and other laboratories have revealed the involvement of several major complex molecular signaling pathways in feather morphogenesis. In general the order of appearance of these molecules is FGF4, BMP4 → SHH, Wnt-7a → Notch-1, Serrate-1 and Delta-1 → Msx-1, -2 → Hox, NCAM (Song et al., 1996; Jung et al., 1998; Ting-Berreth et al., 1996; Widelitz et al., 1999; Chen et al., 1997; Noveen et al., 1995; Chuong et al., 1990, Chuong and Edelman, 1985; Chang et al., 2004a). The role for several molecules in feather morphogenesis is shown schematically in Fig. 4.
Scales and feathers
While testing the effect of mis-expression of these morphogenesis related molecules, we were able to transform avian scale epidermis into feathers by expressing a constitutively active form of β-catenin that was transduced from the replication competent avian sarcoma virus (RCAS). In normal feather development, β-catenin is first all over, then segregates into individual primordia. During that process, they become stronger in the primordia area and absent in the interprimordial area. In scales, β-catenin is weak and diffuse. So we wondered whether enhanced expression of β-catenin in the scale epidermis may make them progress into feathers. Experiments indicate that this is indeed the case (Widelitz et al., 2000). Activation of the delta pathway and suppression of the BMP pathway in scales also can induce some feathery scales (Crowe and Niswander, 1998; Zhou and Niswander, 1996). These molecular pathways are likely to intersect and work in concert during the conversion of scales to feathers. We speculate that similar, but not necessarily identical, molecular processes may have occurred about 150-175 mya during avian evolution to initiate the formation of ancestral feathers.
Barbs and rachis
To investigate the molecules involved in feather branching, we looked for genes with expression patterns suggesting that they might be involved in this process. BMP4 was first expressed in the dermal papilla and overlying pulp, but later switched to the barb ridges in the ramogenic zone. BMP2 was expressed in the marginal plate but later switched to the barb plate. Noggin, a BMP antagonist, was expressed as a gradient in the pulp, with highest expression levels found at the ramogenic zone. To further explore the role of this pathway in branching morphogenesis, we used the RCAS retrovirus to deliver Noggin to regenerating feather follicles of modern chickens (Yu et al., 2002). The resulting rachis was split into smaller ones. Retroviral mediated expression of BMP4 to the regenerating follicles produced feathers with a much thicker rachis. These data suggest that the BMP pathway is involved in specifying keratinocyte fates during feather branching. Furthermore, SHH in the marginal plate is important for the growth control of barb ridges and apoptosis of marginal plate epithelia (Fig. 4; Yu et al., 2002; Chang et al., 2004b).
Because of the possibility to access feather stem cells, to change feather phenotypes with gene mis-expression, and to link molecular pathways with feather forms, the feather is one of the best models for Evo-Devo research (Widelitz et al., 2003).
4.3 Chicken teeth?
Mesozoic birds had teeth that were lost in the evolution of modern beaks. We wondered whether latent molecular signals specifying tooth development were retained by modern birds (Chuong et al., 2001). In the oral mucosa of modern chickens, there is still formation of a dental lamina, but it soon degenerates, which suggested that the ancestral molecular mechanisms might still exist. Some of these latent signals were revealed by in situ hybridization, which indicated that the chicken oral mucosa expressed Pitx2, Pax9, and FGF8, but not Bmp4, Msx1, and Msx2. All of these genes are expressed in the mouse oral mucosa and are considered to be essential for tooth formation. In fact, epithelial signaling to the mesenchyme involves a BMP4 → Msx1 → BMP4 pathway (Chen et al., 1996). Knockout mice lacking Msx-1 and Msx-2 fail to grow teeth (Zhao et al., 2000). It is possible that during beak evolution a defect in the BMP4 → Msx1 → BMP4 pathway developed which led to the loss of teeth from modern birds. To test this theory, our lab and collaborators tried to rescue tooth odontogenesis from the chicken oral mucosa by releasing BMP4 from beads (Chen et al., 2000). BMP4 did induce the expression of Msx1 and Msx2 from the chicken oral mesenchyme. FGF released from beads in a similarly designed experiment had an even greater effect. The effect was even greater still when applied to dorsal skin feather producing mesenchyme (Chen et al., 2000). It is difficult to be sure that these skin appendages were truly teeth since there is no chicken tooth marker. However, these experiments clearly show that oral mucosa epithelia are competent to form follicle like structures.
Experiments using recombination to form a chimera of mouse dental mesenchyme with chicken oral mucosa led to the formation of dental like structures which are reported to even express the enamel gene (Kollar and Fisher, 1980) - although the expression of enamel may result from contamination with mouse ectodermal cells. When the mouse neural tube was transplanted to chick embryos to replace the chick neural crest cells, the mouse/chick chimeras partially recovered the ability to form teeth, but these teeth did not express enamel (Mitsiadis et al, 2003). These data indicate that the avian oral epithelium still reserves competence to form tooth like structures. Does this mean it is the avian neural crest-derived mesenchyme that loses odontogenic capacity? Not quite. Mouse odontogenic epithelium was able to induce molecular changes from chicken mandibular mesenchyme that were similar to those of mouse mandibular mesenchyme (Wang et al., 1998). Thus, many of the signaling members are there, but the epithelial-mesenchymal interactions for chicken tooth formation were suppressed by some means during evolutionary loss of avian teeth.
4.4 Morpho-regulation of ectodermal organs in transgenic mice: Variations or pathology?
Ectodermal organs result from epithelial mesenchymal interactions and keratinocytes are guided to build the organs through induction, morphogenesis, differentiation, and regenerative stages. Different ectodermal organs are considered as variations upon a common theme (Fig. 5A, Chuong, 1998). The many apparently epithelial organs share the use of these signaling molecular pathways (e.g., Wnt, BMP, FGF, Notch, Shh, etc.) and their differences are variations superimposed on the common theme (Chuong, 1998; Chuong and Homberger, 2003). Along this logic, if we imbalance one of fundamental signaling pathways, there should be significant consequences in multiple ectodermal organs in a spatio-temporal dependent manner. We produced a K14-Noggin transgenic mouse to modulate BMP activity and test the extent of this hypothesis (Plikus et al., 2004). We observed thickened skin epidermis, increased hair density, altered hair types, faster anagen re-entry, and formation of compound vibrissa follicles. In the distal limb, there were agenesis and hypogenesis of claws, reduced footpads, and trans-differentiation of sweat glands into hairs. The size of external genitalia increased in both sexes, but they remained fertile (Fig. 5B).
We conclude that modulation of BMP activity can affect the number of ectodermal organs by acting during induction stages, influence the size and shape by acting during morphogenesis stages, change phenotypes by acting during differentiation stages, and facilitate new growth by acting during regeneration stages. Therefore during organogenesis, BMP antagonists can produce a spectrum of phenotypes in a stage-dependent manner by adjusting the level of BMP activity. Should these be considered phenotypic variations or pathological changes?
The concept of “morpho-regulation” implies that morphogenetic processes can be modulated by morphological regulators that lead to changes of morphological phenotypes in development and in evolution. Since the levels of “morpho-regulators” can be adjusted physiologically, they provide means for modulating the morphology of organs without drastic changes. Modulating the pliable BMP pathway as an example, produced changes that are still considered “abnormal” because they indeed significantly deviate from the normal average phenotypes. They may not be really “abnormal” since they are still functional and may be more adaptive in case the environment changes. In the context of evolution, the term “phenotypic plasticity” is used to describe the ability of a quantitative phenotype shift. At the level of species, it may be based on the selection from a spectrum of phenotypic variations based on environmental changes. Examples are seen in the different densities and length of hairs observed in mountain cats, dogs, oxen, etc. from temperate or extremely cold areas found in arctic or high mountain regions, or the shift of finch beak shapes in accord with climate changes in the Galapagos islands. Variations in the number and size of integument appendages can be used to generate a spectrum of variable animal phenotypes that may work as substrates for selection and become advantageous when environments change.
5. Hypothetical models in the Evo-Devo of amniote integument appendages
The origin and early selective history of feather and hair is not so clear. Avian feathers and mammalian hairs are ectodermal structures containing keratin that probably evolved from keratinized epidermal scale in a common reptilian ancestor of mammals and birds (Sharpe, 2001).
5.1 From reptile skin to avian feathers
Reptilian scales and avian feathers have been described as homologous structures (see Lucas and Stettenheim. 1972). Reptile scales, avian scales, and avian feathers are all made from epithelial – mesenchymal interactions. The epidermal cell populations that make up the feathers and scales are similar (Alibardi and Thompson, 2001; Sawyer et al, 2003a), suggesting that the epidermal appendages of reptiles and birds evolved through the modification of epidermal cell populations. Spatial and temporal changes of a gene's expression regulated pattern formation and created skin appendage diversity (Noveen et al., 1998). The similarity and difference among reptile scales, avian scales and avian feathers are listed in Table 2.
There are some fundamental differences in the developmental processes between scales and feathers. Scales do not form follicular structures. Proliferation in avian scales and reptilian scales is more diffuse (Tanaka and Kato, 1983; Alibardi, 1996), so the scales thicken and only elongate slightly. The mature scales are made of an epithelial shell and a mesenchymal core. The outside of the epithelial shell is the suprabasal layer. Studies of molecular expression patterns showed that at the early short bud stage, avian scutate scales, avian feathers and alligator scales share the conserved Shh and Bmp2 polarity pattern (Harris et al, 2002). The conservation of Shh/Bmp2 signaling in the formation between these two integument appendages suggests that the early initiation process of these skin appendages is similar, even if they undergo different differentiation processes to form different skin appendages.
Feathers have a different and complex topological organization. They initially start to proliferate from the tip of feather buds. Therefore, feather buds protrude out first. As the buds elongate, the localized proliferation zone gradually shifts through the shaft and localizes proximally to the base of the feather (Chondankar et al., 2003). In the meantime, epidermis surrounding the feathers starts to invaginate into the dermis to form a follicular wall. The dermal papilla is situated at the base of the follicle, inducing the epithelial collar above to continue proliferation. This allows for continual growth, and facilitates feather cycling as stem cells would be protected in the follicle. During development, the feather filament is a cylindrical structure, with the pulp inside. Toward the distal end, epithelial cells in the ramogenic zone start to differentiate into barb and rachidial ridges which form the rachis and branches (barbs and barbules). Marginal plate cells between barb ridges, pulp epithelium and pulp in the filament center later degenerate to allow the opening of the feather filament cylinder into a two dimensional vane. This vane is made of epithelial cells only and one side is toward the original basal layer (although this basal layer has become the marginal plate and the pulp epithelium, and disappears when the feather vane opens), while the other side is toward the supra-basal layer.
A long held view is that avian feathers evolved from reptile scales. It was suggested that reptilian scales first underwent elongation, later through etching of the elongated scales to produce the branched feather vanes, and finally the inter-woven pennaceous feather barbs became plumulaceous (Regal, 1975). Thus the order of formation is long bilaterally symmetric feather → rachis → pennaceous vane → plumulaceous barbs → radially symmetric downy feathers (Fig. 6B). A “non-avian feather” was reported for the Triassic archosaur, Longisquama (Jones et al., 2000), which supported this hypothesis. In the dorsal midline, Longisquama has a series of paired elongated integument appendages that form branches (Zhang and Zhou, 2000). These branches are perpendicular to the main axis and look like branched scales depicted by Regal (1975). However, a different approach has been taken to investigate skin appendage evolution. From the developmental and molecular studies, it has been proposed that the order of formation is radially symmetric feathers → bilaterally symmetric plumulaceous feathers → bilaterally symmetric vanes → bilaterally asymmetric vanes (Chuong et al., 2000; Prum, 1999; Yu et al., 2002) (Fig. 6B).
Fig. 6. Comparison of the origin and evolution of feathers and hairs from scales.
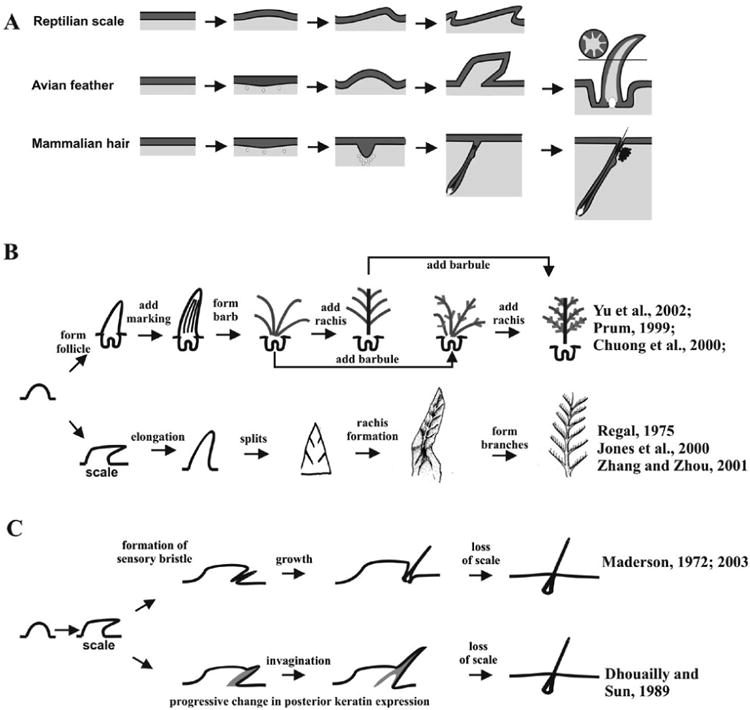
Hypothetical models of amniote skin appendage evolution. A) Comparative developmental processes in reptile scale, avian feathers and mammalian hair. B) Two possible models for the evolution of feathers. Experiments show that the barb - rachis model is correct. C) Models for the evolution of hairs.
5.2 From reptile skin to mammalian hair
Hair does not fossilize well and therefore is not abundant in the fossil record. Therefore the paleontological record did not preserve the evolutionary steps taken from reptilian scale to mammalian hair. The comparison of the embryological development of modern scales and hair showed that hair and scale have taken different developmental processes (Fig. 6A). The similarities and differences between the development of reptilian scale and mammalian hair are listed in Table 2. Unlike reptiles and birds that have both α and β-keratogenesis, with α-keratogenic tissue providing a barrier to water loss and an overlying β-keratogenic layer providing mechanical stiffness to the skin, early theropsid amniotes, including mammalian ancestors, lost β-keratogenic tissue. Their α-keratogenic epidermis was toughened by the mammalian-type HRP (Maderson, 2003).
The Synapsids, a group of reptiles, were the potential ancestors of mammals (Kemp, 1982; Rowe, 1988). They emerged in the Palaeozoic, earlier than 300 myr ago, ruled the earth for 120 myr and then went extinct. Some early Synapsids could have been ectothermic because of the morphological trait of a large “sail” on the dorsum. This sail was composed of elongated spines that were grooved to accommodate blood vessels (Rowe, 1988). The sail could have aided early synapsids to increase or decrease body temperature faster than similarly-sized ectotherms. Theoretically, early sailed synapsids could have developed hair. The transition from ectothermic reptiles with scales to endothermic mammals with hair over 150 mya needed a lot of adaptation, including bone structures, soft tissues, cellular and molecular processes.
A lack of fossil evidence and transition forms between scale and hair at the present time resulted in few proposals (theories) on the origin of hair. Maderson (1972; 2003) hypothesized that hairs arose from reptilian sensory appendages of the mechano-receptor type that were located in the hinge region (Fig. 6C). The mechano-sensory role of the protohairs has been progressively replaced by their thermal insulatory function and contributed to thermoregulatory behavior. They may have evolved spatially patterned sensory protohairs. The multiplication of protohairs may have been caused by mutations leading to increased hair density that can help improve mechanical protection and insulatory function. According to this proposal, certain mutations in the molecular pathways involved in appendage patterning resulted in the expansion of sensory bristles throughout the body of the animal. Proto-pelage provided mechanical protection to fragile α-keratogenic epidermis, made the skin more resistant to abrasion and allowed the Theropsids to move into new terrestrial niches. When mammals moved into a nocturnal niche, proto-hairs started playing an important role in insulation, aiding endothermy. From then on the insulatory factor became a primary driving force in hair evolution. This theory provided good reasoning for behavioral thermoregulation to be initiated and insulation to be a secondary driving force in hair evolution.
Dhouailly and Sun (1989) have proposed an alternative model. In analyzing filiform papillae, they found the inner surface expresses hair keratin and out surface expresses epidermal keratin. By comparing the topology and keratin types of scales, hairs and filiform papillae, they suggest that invaginations in the inner surface of the scale epidermis may lead to a transformation of the scale structure from a hemi-cylindrical form to the more completely cylindrical structure found in hairs. This progression could have been the origin of the evolution novelty of hair follicle invagination (Fig. 2, 7). Recently, the major hair morphogenesis signaling molecules such as Shh, BMP2, 4, etc., are all expressed during the formation of tongue papilla (Jung et al., 1999), corroborating the notion that they are epithelial appendages sharing some history.
Fig. 7. A hypothetical Evo-Devo Space.
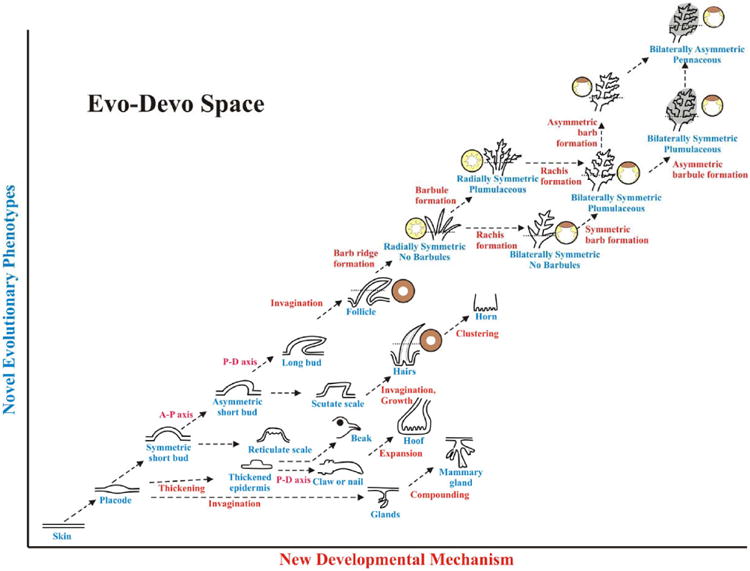
X coordinate represents new developmental mechanism. Y coordinate represent new phenotypes. They all start from a flat layer of epidermis. Those in the right upper quadrant used more novel developmental mechanisms and are more complex. Arrows indicated possible topological transformation from one form to the other, not necessary indicate the evolutionary process. We are working to reveal the molecular basis of those arrows. The processes are in red, and the names of the forms are in blue. Yellow circle is the cross section of developing feather follicles with brown indicating the position of rachis. For comparison, the stage correspond to those used in Prum, 1999 is indicated in (green). Revised from Chuong et al., 2001.
6. Conclusion
Here we take a survey of the evolution of integument appendages of the amniote and try to explain them by the addition of novel developmental mechanisms. For the reptile integument, the major achievement is the formation of an effective barrier that allows reptiles to live on the land freely. The formation of skin folds progresses into scales that are effective in protection and defense. The invaginations of epidermis lead to the independent “invention” of hair follicles and feather follicles. The follicular design allows stem cells to be protected in the dermis and the positioning of a localized TA growth zone in the proximal end of the appendage allows its unlimited growth. Both are used in maintaining endothermy, a more efficient metabolic life style. To gain the increased number of appendages for effective thermo-regulation, Maderson (1972, 2003) proposed a multiplication of appendage units in proto-mammals. In avian precursors, barb branching and barbule formation were used initially to produce warming downy feathers. Subsequent fusion of barbs to form a rachis, and the differentiation and interlocking of the proximal and distal barbules to form feather vanes have allowed birds to fly. By changing molecular circuits, we and others have explored the molecular and cellular basis underlying these morphogenetic changes through laboratory research.
We have learned that a single piece of epidermis can be folded to form different forms of epithelial organs that differentiate differently (Fig. 7). They represent variations of epithelial- mesenchymal interactions superimposed on a common theme (Chuong, 1998; Chuong et al., 2000). The diverse integument appendages existing in reptiles, birds, and mammals were not achieved in one step, but represent millions of years of trial and error by Nature. We are just at the beginning of understanding these processes. As we learn more about how molecular pathways work in the model organisms, we need to pursue more comparative studies to appreciate how Nature formed myriad structures in many diverse ways in the wild, how the integument appendages interface with other systems to perform a physiological function for the whole organism (e.g., flight), and how genes interact with the environment. Only with these endeavors, can we truly understand the essence of integument biology (Chuong and Homberger, 2003).
Acknowledgments
We thank Dr. Dhouailly for the very helpful comments during the preparation of this manuscript. This work was supported by grants from NIH, NSF (CMC) and NCI (RW). LH was supported by grants from the NSP (4982020) and IVPP. SS was supported by the Royal Thai Government Scholarship.
References
- Alibardi L. Scale morphogenesis during embryonic development in the lizard Anolis lineatopus. J Anat. 1996;188:713–25. [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J Exp Zool Part B Mol Dev Evol. 2003;298:12–41. doi: 10.1002/jez.b.24. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Maderson PFA. Observations on the histochemistry and ultrastructure of the epidermis of the Tuatara, Sphenodon punctatus (Spehnodontida, Lepidosauri, Reptilia): a contribution to an understanding of the lepidosaurian epidermal generation and the evolutionary origin of the squamate shedding complex. J Morphol. 2003;256:111–33. doi: 10.1002/jmor.10079. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Sawyer RH. Immunocytochemical analysis of beta keratins in the epidermis of chelonians, lepidosaurians, and archosaurians. J Exp Zool. 2002;293:27–38. doi: 10.1002/jez.10145. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Thompson MB. Fine structure of the developing epidermis in the embryo of the American alligator (Alligator mississippiensis, Crocodilia, Reptilia) J Anat. 2001;198:265–82. doi: 10.1046/j.1469-7580.2001.19830265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L, Thompson MB. Keratinization and ultrastructure of the epidermis of late embryonic stages in the alligator (Alligator mississippiensis) J Anat. 2002;201:71–84. doi: 10.1046/j.1469-7580.2002.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Autumn K, Liang YA, Hsieh ST, Zesch W, Chan WP, Kenny TW, Fearing R, Full RJ. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–5. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- Baden HP, Maderson PF. Morphological and biophysical identification of fibrous proteins in the amniote epidermis. J Exp Zool. 1970;174:225–32. doi: 10.1002/jez.1401740211. [DOI] [PubMed] [Google Scholar]
- Balcomb K, Minasian S. The World's Whales Smithsonian Books. W. W. Norton; New York: 1984. [Google Scholar]
- Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J Exp Zool Part B Mol Dev Evol. 2003;298:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Biology of the integument. New York: Springer Verlag; 1986. [Google Scholar]
- Blanchet S, Favier B, Chevalier G, Kastner P, Michaille JJ, Chambon P, Dhouailly D. Both retinoic acid receptors alpha (RARalpha) and gamma (RARgamma) are able to initiate mouse upper-lip skin glandular metaplasia. J Invest Dermatol. 1998;111:206–12. doi: 10.1046/j.1523-1747.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool Part B Mol Dev Evol. 2003;298:164–80. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- Bragulla H, Hirschberg RM. Horse hooves and bird feathers: Two model systems for studying the structure and development of highly adapted integumentary accessory organs--the role of the dermo-epidermal interface for the micro-architecture of complex epidermal structures. J Exp Zool Part B Mol Dev Evol. 2003;298:140–51. doi: 10.1002/jez.b.31. [DOI] [PubMed] [Google Scholar]
- Carpenter PW, Davies C, Lucey AD. Hydrodynamics and compliant walls: Does the dolphin have a secret? Current Science. 2000;79:758–65. [Google Scholar]
- Chaloin-Dufau C, Pavitt I, Delorme P, Dhouailly D. Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial Cell Biol. 1993;2:120–5. [PubMed] [Google Scholar]
- Chang CH, Jiang TX, Lin CM, Burrus L, Chuong CM, Widelitz RB. Distinctive Phenotypes Generated by WNT Member misexpression During Hierarchical Morphogenesis of Dermis, Skin Regions and Individual Feathers. Mech Develop. 2004a;121:157–71. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting Skin Appendages Out of Epidermal Layers Via Temporally and Spatially Regulated Apoptotic Events. J Invest Dremato. 2004b doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RE. Hair, wool, quill nail, claw, hoof, and horn. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument, Vol 2, Vertebrates. Springer - Verlag; New York: 1986. pp. 293–312. [Google Scholar]
- Chatterjee S. The Rise of Birds. John Hopkins University Press; Baltimore, MD: 1997. [Google Scholar]
- Chen CW, Jung HS, Jiang TX, Chuong CM. Asymmetric expression of notch/Delta/Serrate is associated with the anterior-posterior axis of feather buds. Dev Biol. 1997;188:181–87. doi: 10.1006/dbio.1997.8643. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–44. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Chen P, Dong Z, Zhen S. An exceptionally well preserved theropod dinosaur from the Yixian Formation of China. Nature. 1998;391:147–52. [Google Scholar]
- Chen YP, Zhang Y, Jiang TX, Barlow A, Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathway in Aves. Proc Natl Acad Sci. 2000;97:10044–49. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe LM. The First 85 million years of Avian Evolution. Nature. 1995;378:349–55. [Google Scholar]
- Chodankar R, Chang CH, Yue Z, Jiang TX, Suksaweang S, Burrus L, Chuong CM, Widelitz R. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:20–6. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience, Austin, TX: Landes Bioscience; 1998. [Google Scholar]
- Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages. Curr Opin Genet Dev. 2000;10:449–56. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell-adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J Cell Biol. 1985;101:1027–43. doi: 10.1083/jcb.101.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Homberger DG. Development and evolution of the amniote integument: current landscape and future horizon. J Exp Zool Part B Mol Dev Evol. 2003;298:1–11. doi: 10.1002/jez.b.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110:1021–30. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- Chuong CM. The making of a feather: homeoproteins, retinoids and adhesion molecules. Bioessays. 1993;15:513–21. doi: 10.1002/bies.950150804. [DOI] [PubMed] [Google Scholar]
- Chuong CM, Hou L, Chen PJ, Wu P, Patel N, Chen Y. Dinosaur's feather and chicken's tooth? Tissue engineering of the integument. Eur J Dermatol. 2001;11:286–92. [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zoolog Part B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol. 2003;48:1–14. doi: 10.1016/s0003-9969(02)00208-x. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crowe R, Niswander L. Disruption of scale development by Delta-1 misexpression. Dev Biol. 1998;195:70–4. doi: 10.1006/dbio.1997.8844. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species: A facsimile of the first edition. Harvard Univ. Press; Cambridge: 1859. 1975. [Google Scholar]
- Dhouailly D, Maderson PFA. Ultrastructural observations on the embryonic development of the integument of Lacerta muralis (Lacertilia, Reptilia) J Morphol. 1984;179:203–28. doi: 10.1002/jmor.1051790302. [DOI] [PubMed] [Google Scholar]
- Dhouailly D. Formation of cutaceous appendages in dermo-epidermal recombinaitons between reptiles, birds and mammals. Wilhelm Roux' Arch Entwicklungsmech Org. 1975;177:323–40. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions during morphogenesis of cutaneous appendages in amniotes. Front matrix Biol. 1977;4:86–121. [Google Scholar]
- Dhouailly . Specification of feather and scale patterns. In: Malacinski GM, Bryant SW, editors. Pattern formation. Mac millan Pub; New York, London: 1984. pp. 581–601. [Google Scholar]
- Dhouailly D, Hardy MH, Sengel P. Formation of feathers on chick foot scales: a stage-dependent morphogenetic response to retinoic acid. J Embryol Exp Morphol. 1980;58:63–78. [PubMed] [Google Scholar]
- Dhouailly D, Sun TT. The mammalian tongue filiform papillae: a theoretical model for primitive hairs. In: van Neste D, LaCapelle JM, Antoine JL, editors. Trends in human hair growth and Alopecia research. Kluwer Acad. Pub; Boston: 1989. pp. 29–34. [Google Scholar]
- Feduccia A. The Origin and Evolution of Birds. 2nd. Yale University Press; New Haven, CT: 1999. [Google Scholar]
- Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D. Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development. 2000;127:5487–95. doi: 10.1242/dev.127.24.5487. [DOI] [PubMed] [Google Scholar]
- Fraser RD, Parry DA. The molecular structure of reptilian keratin. Int J Biol Macromol. 1996;19:207–11. doi: 10.1016/0141-8130(96)01129-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–53. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- Gill FB. Ornithology. 2nd. Freeman; New York, NY: 1994. [Google Scholar]
- Goldman AS. Evolution of the mammary gland defense system and the ontogeny of the immune system. J Mammary Gland Biol Neoplasia. 2002;7:277–89. doi: 10.1023/a:1022852700266. [DOI] [PubMed] [Google Scholar]
- Grant PR. In: Ecology and evolution of Darwin's finches. Grant PR, editor. Princeton Univ. Press; Princeton, NJ: 1986. pp. 1–492. [Google Scholar]
- Gregg K, Wilton SD, Parry DA, Rogers GE. A comparison of genomic coding sequences for feather and scale keratins: structural and evolutionary implications. EMBO J. 1984;3:175–8. doi: 10.1002/j.1460-2075.1984.tb01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg K, Rogers GE. Feather keratin: composition, structure and biogenesis. In: Bereiter-Hahn J, editor. Biology of the integument Vol 2 Vertebrates. Springer Verlag; New York: 1986. pp. 666–94. [Google Scholar]
- Grossl NA. Supernumerary breast tissue: historical perspectives and clinical features. South Med J. 2000;93:29–32. [PubMed] [Google Scholar]
- Grüneberg H. The glandular aspects of the Tabby syndrome in the mouse. J Embryol Exp Morph. 1971;25:1–19. [PubMed] [Google Scholar]
- Haake AR, Konig G, Sawyer RH. Avian feather development: relationships between morphogenesis and keratinization. Dev Biol. 1984;106:406–13. doi: 10.1016/0012-1606(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Hamrick MW. Evolution and development of mammalian limb integumentary structures. J Exp Zool Part B Mol Dev Evol. 2003;298:152–63. doi: 10.1002/jez.b.32. [DOI] [PubMed] [Google Scholar]
- Hardy MH, Bellows CG. The stability of vitamin A-induced metaplasia of mouse vibrissa follicles in vitro. J Invest Dermatol. 1978;71:236–41. doi: 10.1111/1523-1747.ep12515093. [DOI] [PubMed] [Google Scholar]
- Hardy MH, Dhouailly D, Torma H, Vahlquist A. Either chick embryo dermis or retinoid-treated mouse dermis can initiate glandular morphogenesis from mammalian epidermal tissue. J Exp Zool. 1990;256:279–89. doi: 10.1002/jez.1402560307. [DOI] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–76. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Homberger DG, de Silva KN. The role of mechanical forces on the patterning of the avian feather-bearing skin: A biomechanical analysis of the integumentary musculature in birds. J Exp Zool Part B Mol Dev Evol. 2003;298:123–39. doi: 10.1002/jez.b.30. [DOI] [PubMed] [Google Scholar]
- Hou LH, Chuong CM, Yang A, Zeng XL, Hou JF. Fossil Birds of China. Yunnan Science and Technology; China: 2003. [Google Scholar]
- Hou L, Chiappe LM, Zhang F, Chuong CM. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91:22–5. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LH, Chuong CM, Yang A, Zeng XL, Hou JF. Fossil Birds of China. Yunnan Science and Technology; China: 2003. [Google Scholar]
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl 1):437–81. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Keranen SV, Thesleff I. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc Natl Acad Sci U S A. 2000;97:14444–8. doi: 10.1073/pnas.97.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Currie PJ, Norell MA, Ji SA. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–61. [Google Scholar]
- Ji Q, Norell MA, Gao KQ, Ji SA, Ren D. The distribution of integumentary structures in a feathered dinosaur. Nature. 2001;410:1084–8. doi: 10.1038/35074079. [DOI] [PubMed] [Google Scholar]
- Johnston B, Rose J. Role of prolactin in regulating the onset of winter fur growth in mink (Mustela vison): A reconsideration. J Exp Zool. 1999 Sep 1;284(4):437–44. doi: 10.1002/(sici)1097-010x(19990901)284:4<437::aid-jez10>3.0.co;2-x. 1999. [DOI] [PubMed] [Google Scholar]
- Jones TD, Ruben JA, Martin LD, Kurochkin EN, Feduccia A, Maderson PF, Hillenius WJ, Geist NR, Alifanov V. Nonavian feathers in a late Triassic archosaur. Science. 2000;288:2202–5. doi: 10.1126/science.288.5474.2202. [DOI] [PubMed] [Google Scholar]
- Jung HS, Francis-West F, Widelitz RB, Jiang TX, Ting S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: Implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Jung HS, Oropeza V, Thesleff I. Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech Dev. 1999;81:179–82. doi: 10.1016/s0925-4773(98)00234-2. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Prin F, Thelu J, Dhouailly D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 1997;210:274–87. doi: 10.1002/(SICI)1097-0177(199711)210:3<274::AID-AJA8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kemp TS. Mammal-like Reptiles and the Origin of Mammals. Academic press; New York: 1982. p. 363. [Google Scholar]
- Kim JY, Mochizuki T, Akita K, Jung HS. Morphological evidence of the importance of epithelial tissue during mouse tongue development. Exp Cell Res. 2003;290:217–26. doi: 10.1016/s0014-4827(03)00319-7. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–5. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kuwahara Y, Kondo M, Naruse K, Mitani H, Wakamatsu Y, Ozato K, Asakawa S, Shimizu N, Shima A. The medaka rs-3 locus required for scale development encodes ectodysplasin-A receptor. Curr Biol. 2001;11:1202–6. doi: 10.1016/s0960-9822(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Lako M, Armstrong L, Cairns PM, Harris S, Hole N, Jahoda CA. Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci. 2002;115:3967–74. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- Landmann L. The Skin of Reptiles: Epidermis and Dermis. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument, Vol 2, Vertebrates. Springer - Verlag; New York: 1986. pp. 150–187. [Google Scholar]
- Lane EB, Wilson CA, Hughes BR, Leigh IM. Stem cells in hair follicles. The molecular and structural biology of hair. Ann N Y Acad Sci. 1991;642:197–213. doi: 10.1111/j.1749-6632.1991.tb24388.x. [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Winter H, Praetzel S, Beckhaus U, Rackwitz HR, Schweizer J. The catalog of human hair keratins. I. Expression of the nine type I members in the hair follicle. J Biol Chem. 1999;274:19874–84. doi: 10.1074/jbc.274.28.19874. [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Winter H, Praetzel S, Schweizer J. The catalog of human hair keratins. II. Expression of the six type II members in the hair follicle and the combined catalog of human type I and II keratins. J Biol Chem. 2001;276:35123–32. doi: 10.1074/jbc.M103305200. [DOI] [PubMed] [Google Scholar]
- Leatherwood SL, Reeves RR. The Sierra Club Handbook of Whales and Dolphins. Sierra Club Books; San Francisco: 1983. [Google Scholar]
- Loredo GA, Brukman A, Harris MP, Kagle D, Leclair EE, Gutman R, Denney E, Henkelman E, Murray BP, Fallon JF, Tuan RS, Gilbert SF. Development of an evolutionarily novel structure: fibroblast growth factor expression in the carapacial ridge of turtle embryos. J Exp Zool. 2001;291:274–81. doi: 10.1002/jez.1103. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Sttetenheim PR. Agriculture Handbook 362 Agricultural Research Services. Washington, DC: US Department of Agriculture; 1972. Avian Anatomy Integument. [Google Scholar]
- Lynch LJ, Robinson V, Anderson CA. A scanning electron microscope study of the morphology of rhinoceros horn. Aust J Biol Sci. 1973;26:395–9. doi: 10.1071/bi9730395. [DOI] [PubMed] [Google Scholar]
- Maderson PFA. The structure and development of the squamate epidermis. In: Lyne AG, Short BF, editors. The biology of the skin and hair growth. Angus and Robertson; Sidney: 1965. pp. 129–53. [Google Scholar]
- Maderson PFA. When? Why? And How? Some speculations on the evolution of the vertebrate integument. Am Zool. 1972;12:159–71. [Google Scholar]
- Maderson PFA. Mammalian skin evolution: a reevaluation. Exp Dermatol. 2003;12:233–236. doi: 10.1034/j.1600-0625.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- Maderson PFA, Sawyer RH. Scale embryogenesis in birds and reptiles. The Anatomical Record. 1979;193:609. [Google Scholar]
- Maderson PFA, Rabinovitz T, Tandler B, Alibardi L. Ultrastructural contributions to an understanding of the cellular mechanisms in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J Morphol. 1998;236:1–24. doi: 10.1002/(SICI)1097-4687(199804)236:1<1::AID-JMOR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Yoshizato K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair Regen. 1998;6:524–30. doi: 10.1046/j.1524-475x.1998.60605.x. [DOI] [PubMed] [Google Scholar]
- Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–65. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–705. doi: 10.1101/gad.891401. Related Articles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev 2003. 2003;14:211–24. doi: 10.1016/s1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc Natl Acad Sci U S A. 2003;100:6541–45. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J. Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet. 1999;22:366–369. doi: 10.1038/11937. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The Developing Human: Clinically Oriented Embryology. 6th. W.B. Saunders Company; Philadelphia: 1998. pp. 514–521. [Google Scholar]
- Nakamura M, Sundberg JP, Paus R. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: annotated tables. Exp Dermatol. 2001;10:369–90. doi: 10.1034/j.1600-0625.2001.100601.x. [DOI] [PubMed] [Google Scholar]
- Norell M, Ji Q, Gao K, Yuan C, Zhao Y, Wang L. Palaeontology: ‘modern’ feathers on a non-avian dinosaur. Nature. 2002;416:36–37. doi: 10.1038/416036a. [DOI] [PubMed] [Google Scholar]
- Noveen A, Jiang TX, Ting-Berreth SA, Chuong CM. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. J Invest Dermatol. 1995;104:711–9. doi: 10.1111/1523-1747.ep12606960. [DOI] [PubMed] [Google Scholar]
- Noveen A, Hartenstein V, Chuong CM. Gene networks and supernetworks: evolutionarily conserved gene interactions. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes Bioscience; 1998. pp. 371–91. [Google Scholar]
- Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7:225–52. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- O'Guin WM, Sawyer RH. Avian scale development. VII. Relationships between morphogenetic and biosynthetic differentiation. Dev Biol. 1982;89:485–92. doi: 10.1016/0012-1606(82)90336-0. [DOI] [PubMed] [Google Scholar]
- Oliver RF. Ectopic regeneration of whiskers in the hooded rat from implanted lengths of vibrissa follicle wall. J Embryol Exp Morphol. 1967;17:27–34. [PubMed] [Google Scholar]
- Patterson B. Pholidota and Tubulidentata. In: Maglio VJ, Cooke HBS, editors. Evolution of African mammals. Harvard University Press; Cambridge, Massachusetts: 1978. [Google Scholar]
- Peaker M. The mammary gland in mammalian evolution: a brief commentary on some of the concepts. J Mammary Gland Biol Neoplasia. 2002;7:347–53. doi: 10.1023/a:1022860902083. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic Protein Pathway. Am J Pathol. 2004;164:1099–114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pough FH, Andrews RM, Cadle JE, Crump ML, Savitzky AH, Wells KD. Herpetology. 2nd. Prentice-Hall, Inc; Upper Saddle River, NJ: 2001. [Google Scholar]
- Presland RB, Gregg K, Molloy PL, Morris CP, Crocker LA, Rogers GE. Avian keratin genes. I. A molecular analysis of the structure and expression of a group of feather keratin genes. J Mol Biol. 1989a;209:549–59. doi: 10.1016/0022-2836(89)90593-7. [DOI] [PubMed] [Google Scholar]
- Presland RB, Whitbread LA, Rogers GE. Avian keratin genes. II. Chromosomal arrangement and close linkage of three gene families. J Mol Biol. 1989b;209:561–76. doi: 10.1016/0022-2836(89)90594-9. [DOI] [PubMed] [Google Scholar]
- Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–95. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- Prum RO, Dyck J. A hierarchical model of plumage: morphology, development, and evolution. J Exp Zool Part B Mol Dev Evol. 2003;298:73–90. doi: 10.1002/jez.b.27. [DOI] [PubMed] [Google Scholar]
- Regal PJ. The evolutionary origin of feathers. Q Rev Biol. 1975;50:35–66. doi: 10.1086/408299. [DOI] [PubMed] [Google Scholar]
- Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–73. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- Rowe T. Definition, diagnosis, and origin of Mammalia. Journal of Vertebrate Paleontology. 1988;8:241–64. [Google Scholar]
- Ruben JA, Jones TD. Selective factors associated with the origin of fur and feathers. Amer Zool. 2000;40:585–96. [Google Scholar]
- Sawyer RH. Avian scale development. I. Histogenesis and morphogenesis of the epidermis and dermis during formation of the scale ridge. J Exp Zool. 1972;181:365–81. [Google Scholar]
- Sawyer RH, Craig KF. Avian scale development. Absence of an “epidermal placode” in reticulate scale morphogenesis. J Morphol. 1977;154:83–93. doi: 10.1002/jmor.1051540106. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Knapp LW, O'Guin WM. The skin of birds: epidermis, dermis and appendages. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument, Vol 2, Vertebrates. Springer - Verlag; New York: 1986. pp. p 194–238. [Google Scholar]
- Sawyer RH, Glenn T, French JO, Mays B, Shames RB, Barnes GL, Jr, Rhodes W, Ishikawa Y. The expression of Beta (β) keratins in the epidermal appendages of reptiles and birds. Amer Zool. 2000;40:530–9. [Google Scholar]
- Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zool Part B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Salvatore BA, Potylicki TT, French JO, Glenn TC, Knapp LW. Origin of feathers: Feather beta (β) keratins are expressed in discrete epidermal cell populations of embryonic scutate scales. J Exp Zool Part B Mol Dev Evol. 2003a;295:12–24. doi: 10.1002/jez.b.5. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Washington LD, Salvatore BA, Glenn TC, Knapp LW. Origin of archosaurian integument appendages: the bristles of the wild turkey beard express feather-type beta keratins. J Exp Zool Part B Mol Dev Evol. 2003b;297:27–34. doi: 10.1002/jez.b.17. [DOI] [PubMed] [Google Scholar]
- Schweitzer MH, Watt JA, Avci R, Knapp L, Chiappe L, Norell M, Marshall M. Beta-keratin specific immunological reactivity in feather-like structures of the cretaceous alvarezsaurid, Shuvuuia deserti. J Exp Zool. 1999;285:146–57. [PubMed] [Google Scholar]
- Sengel P. Recherches experimentales sur la differenciation des germes plumaires et du pigment de la peau de l'embryon de poupes en culture in vitro. Ann Sci Nat Zool. 1958;20:421–514. [Google Scholar]
- Sereno PC. The evolution of dinosaurs. Science. 1999;284:2137–47. doi: 10.1126/science.284.5423.2137. [DOI] [PubMed] [Google Scholar]
- Shames RB, Knapp LW, Carver WE, Washington LD, Sawyer RH. Keratinization of the outer surface of the avian scutate scale: Interrelationship of alpha and beta keratin filaments in cornifying tissue. Cell and Tissue Research. 1989;257:85–92. doi: 10.1007/BF00221637. [DOI] [PubMed] [Google Scholar]
- Shames RB, Knapp LW, Carver WE, Sawyer RH. Region-specific expression of scutate scale type beta keratins in the developing chick beak. J Exp Zool. 1991;260:258–66. doi: 10.1002/jez.1402600215. [DOI] [PubMed] [Google Scholar]
- Sharpe PT. Fish scale development: hair today, teeth and scales yesterday? Current Biology. 2001;11:R751–2. doi: 10.1016/s0960-9822(01)00438-9. [DOI] [PubMed] [Google Scholar]
- Song H, Wang Y, Goetinck PF. Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proc Natl Acad Sci U S A. 1996;93:10246–9. doi: 10.1073/pnas.93.19.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP. Handbook of Mouse Mutations with Skin and Hair Abnormalities. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Tanaka S, Kato Y. Epigenesis in developing avian scales. II. Cell proliferation in relation to morphogenesis and differentiation in the epidermis. J Exp Zool. 1983;225:271–83. doi: 10.1002/jez.1402250210. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–70. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Thibaut S, Collin C, Langbein L, Schweizer J, Gautier B, Bernard BA. Hair keratin pattern in human hair follicles grown in vitro. Exp Dermatol. 2003;12:160–4. doi: 10.1034/j.1600-0625.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–57. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Vincent C, Bontoux M, Le Douarin NM, Pieau C, Monsoro-Burq AH. Msx genes are expressed in the carapacial ridge of turtle shell: a study of the European pond turtle, Emys orbicularis. Dev Genes Ecol. 2003;213:464–9. doi: 10.1007/s00427-003-0347-3. [DOI] [PubMed] [Google Scholar]
- Waite PM, Li L. Unmyelinated innervation of sinus hair follicles in rats. Anat Embryol (Berl) 1993;188:457–65. doi: 10.1007/BF00190140. [DOI] [PubMed] [Google Scholar]
- Wang YH, Upholt WB, Sharpe PT, Kollar EJ, Mina M. Odontogenic epithelium induces similar molecular responses in chick and mouse mandibular mesenchyme. Dev Dyn. 1998;213:386–97. doi: 10.1002/(SICI)1097-0177(199812)213:4<386::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Weiss KM, Stock DW, Zhao Z. Dynamic interactions and the evolutionary genetics of dental patterning. Crit Rev Oral Biol Med. 1998;9:369–98. doi: 10.1177/10454411980090040101. [DOI] [PubMed] [Google Scholar]
- Whitbread LA, Gregg K, Rogers GE. The structure and expression of a gene encoding chick claw keratin. Gene. 1991;101:223–9. doi: 10.1016/0378-1119(91)90415-8. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Chen CW, Stott NS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development. 1999;126:2577–87. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Lu J, Chuong CM. beta-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen JY, Wu P, Yu Z, Chuong CM. Molecular biology of feather morphogenesis: a testable model for evo-devo research. J Exp Zool Part B Mol Dev Evol. 2003;298:109–22. doi: 10.1002/jez.b.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn LK, Winn HE. Wings in the Sea; the Humpback Whale. University Press of New England; Hanover, NH: 1985. [Google Scholar]
- Wu T, Chuong CM. Developmental Biology of Skin Appendages. In: Camacho F, Randall VA, Price VH, editors. Hair Biology and Disorders: Research, Pathology and Management. Martin Dunitz Pub; 2000. pp. 17–37. In press. [Google Scholar]
- Xu X, Tang ZJ, Wang XJ. A therinzinosauroid dinosaur with integumentary structures from China. Nature. 1999a;399:350–4. [Google Scholar]
- Xu X, Wang XL, Wu XC. A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature. 1999b;401:262–6. [Google Scholar]
- Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–8. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Prum RO. Branched integumental structures in Sinornithosaurus and the origin of feathers. Nature. 2001;410:200–4. doi: 10.1038/35065589. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–40. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–60. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol. 1993;101:652–9. doi: 10.1111/1523-1747.ep12371671. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–12. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 2000;290:1955–9. doi: 10.1126/science.290.5498.1955. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Song Y, Zhang X, Zhang Y, Hu Y, Fromm SH, Chen Y. Transgenically ectopic expression of Bmp4 to the Msx1 mutant dental mesenchyme restores downstream gene expression but represses Shh and Bmp2 in the enamel knot of wild type tooth germ. Mech Dev. 2000;99:29–38. doi: 10.1016/s0925-4773(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421:807–14. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- Zhou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–41. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]


