Abstract
Purpose of review
This review summarizes the development and implementation of a large clinical trial, HIV Prevention Trials Network (HPTN) 052, whose initial results were recently presented and published.
Recent findings
A randomized, clinical trial demonstrated that antiretroviral therapy reduces the sexual transmission of HIV in HIV-serodiscordant couples by more than 96%. The logistical challenges in preparing for and conducting such a trial were considerable.
Summary
HPTN 052 required many years of preparation, considerable collaboration between National Institute of Health and six pharmaceutical companies, and careful ongoing consideration of a large number of ethical issues. HPTN 052 revealed the magnitude of benefit when using antiretroviral therapy to prevent the transmission of HIV, and served as proof of a concept. The results have proven central to the development of new global HIV-prevention efforts.
Keywords: antiretroviral therapy, prevention, transmission
INTRODUCTION
The use of antiretroviral therapy (ART) for the treatment of HIV-1 is currently one of the most popular ideas to reduce transmission of HIV [1 – 8]. The strategy has generated a plethora of mathematical models that, for the most part, predict success [9–14,15*,16,17,18**,19 – 21], and several ecological studies that argue that the prevention benefit of ART can already be seen in a few communities where ART usage is high [22,23,24*,25*].
These public health aspirations are truly important. However, policies favoring the use of treatment as prevention have been rapidly moving forward in the absence of a critical piece of information: the degree to which ART actually prevents the transmission of HIV and the durability of the effect(s) of ART for this purpose. The purpose of this article is to review briefly the development and results of a single, multicenter, Phase III, clinical trial designed to ask whether the treatment of HIV infection also prevents the sexual transmission of HIV-1 in HIV-serodiscordant couples.
BIOLOGICAL PLAUSIBILITY
In 2000, researchers from the Rakai (Uganda) project [26] reported that the concentration of HIV in blood plasma could be correlated with HIV transmission probability in HIV-serodiscordant couples. The study concluded that the risk of transmission of HIV with less than 1500 copies of HIV RNA is negligible.
Prior to the Rakai study, however, several reports showed that HIV can also be recovered in genital secretions, as would be predicted based on the sexual transmission of the virus. With the development of the first antiretroviral agent – azidothymidine – interest surfaced in reducing replication of HIV in the genital tract with ART [27]. Many groups working on this problem were focused on developing “treatment as prevention'. Indeed, in 1997, in a study from our group on this matter, we stated, `… antiretroviral therapy, by decreasing levels of HIV-1 in semen, may lower the infectious inoculum of treated men and possibly reduce the likelihood of sexual transmission to uninfected partners (p. 60) [28].
This work led to a series of reports about the concentration of HIV in semen using quantitative culture [29 – 31] and development of a PCR technique for more accurate measurement [32]. With these tools, studies demonstrated that ART reduced the concentration of HIV in both semen [33, 34] and female genital secretions [35]. Evidence also emerged that antiretroviral drugs penetrate the male and female genital tract with different efficiency, [36, 37] helping to explain the sexual transmission of resistant viruses [38 – 41].
HPTN 052: TRIAL DESIGN
Consistent with the Rakai study [26], other observational studies supported the critical importance of viral load in HIV transmission [42]. However, blood viral load never correlates perfectly with genital tract HIV [43 – 45]. In addition, the exact mode of HIV transmission – whether from cell-free or cell- bound virus remains obscure. Although inductive logic suggested that ART that suppresses HIV replication should also reduce HIV transmission, the magnitude and durability of such an effect could not be determined without empirical evidence. In 2000, the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH)-sponsored HIV Prevention Trials Network (HPTN) approved the development of a protocol to study the use of ART to reduce HIV in blood and genital secretions and, thus, prevent the sexual transmission of HIV in HIV-serodiscordant couples.
After careful consideration, the only plausible and ethical design was to randomize HIV-serodiscordant couples into two study arms: early vs. delayed therapy. In the early arm, ART would be offered at CD4 cell counts higher than when ART was recommended by the WHO in the respective countries where the study would take place. In the delayed arm, ART would be offered when the CD4 cell count fell to the general standard of care, that is, below 200 cells/ml in the pilot phase of the study, modified to at or below 250 cells/mm3 in the full trial (Fig. 1). Through a detailed statistical plan, including modeling of the timeframe for both the decline of CD4 cell counts and potential trans- mission events, it was determined that 1750 participants with HIV (and CD4 cell counts between 300 and 500 cells/ml in the pilot phase, and 350 – 550 cells/ml in the full study) and their HIV-negative partners would be needed to answer the primary objective, which was to compare the rates of HIV infection between the two arms (Fig. 1). As already noted, the CD4 cell count inclusion criterion changed over the course of the study. Thirteen clinical sites worldwide participated (nine initially, and four more in 2009 in order to increase the rate of enrollment), starting with a pilot phase in 2005, followed by the initiation of the full study in 2007. A list of the sites, the number of couples enrolled at each site, and the enrollment inclusion/exclusion criteria are shown in Tables 1 and 2 [46**]. The current version of the protocol is available at http://www.hptn.org.
Figure 1.
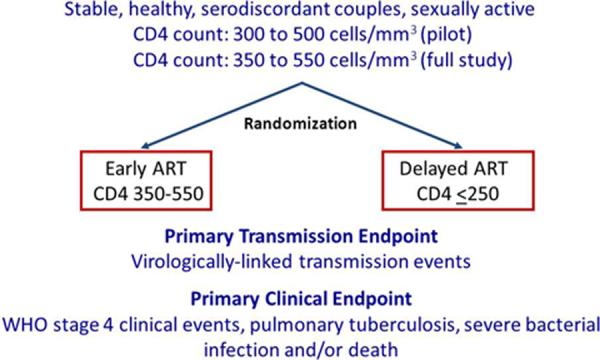
HPTN 052 Study Design
Table 1.
Enrollment of HIV-Serodiscordant Couples by Site
| Site | Enrollment (Number of Couples) |
|---|---|
| Porto Alegre, Brazil | 90 |
| Rio de Janeiro, Brazil | 186 |
| Boston, MA, USA | 2 |
| Chennai, India | 250 |
| Pune, India | 175 |
| Chiang Mai, Thailand | 106 |
| Kisumu, Kenya | 60 |
| Harare, Zimbabwe | 240 |
| Blantyre, Malawi | 230 |
| Lilongwe, Malawi | 251 |
| Gaborone, Botswana | 77 |
| Johannesburg, South Africa | 46 |
| Soweto, South Africa | 50 |
| Total Enrollment | 1763 |
HPTN 052: PRE-IMPLEMENTATION REQUIREMENTS
Having designed a plausible trial, the study faced two difficult challenges. First, NIAID policy in the early 2000s required that US pharmaceutical companies donate drugs for use in the study. We calculated that 1750 discordant couples would be recruited and followed on-study until the last couple completed 5 years of study follow-up, and the statistical plan assumed that every HIV-infected person in the delayed arm would eventually be placed on ART (statistical plan available in the protocol at http://www.hptn.org). These assumptions led to the recognition that large amounts of drug were required to conduct the study successfully, no company would receive actual benefit from the trial because no treatment comparisons were planned, and multiple anti-retroviral combinations would be required for first- and second-line therapy. Through an iterative process, we were able to establish a collaborative agreement with each of the companies listed in Table 3 to provide their drug products. Second, our collaborating partners in the NIH-sponsored AIDS Clinical Trials Group (ACTG) raised concerns that subjects with low CD4 cell count screening out of HPTN 052 required an option for treatment. This finding led to the development and implementation of an inter- national ACTG protocol (ACTG 5175), launched at all participating HPTN 052 sites at the time the HPTN 052 study began [47,48].
Table 3.
Antiretroviral Drug Donations for HPTN 052
| Pharmaceutical Company | Donated Antiretroviral Drug |
|---|---|
| Abbott Laboratories | Kaletra®/Aluvia® (lopinavir/ritonavir) Ritonavir |
| Boehringer-lngelheim Pharmaceuticals, Inc. (BI) | Nevirapine |
| Bristol-Myers Squibb (BMS) | Atazanavir didanosine stavudine efavirenz (US sites) |
| Gilead Sciences, Inc. | Truvada (emtricitabine/tenofovir) Tenofovir |
| GlaxoSmithKline(GSK) | Combivir® (lamivudine/zidovudine) Lamivudine |
| Merck & Co., Inc. | efavirenz (non-US sites) |
Other pre-study implementation requirements included the development of detailed clinical and counseling materials. Regional trainings were conducted over the course of a year on the clinical management of ART and couples risk-reduction counseling. In addition, on-site training on the protocol requirements was conducted at all 13 sites over the course of 2 years.
HPTN 052: ETHICS AND EQUIPOISE
The ethical considerations related to HPTN 052 are substantial and will be the subject of a separate detailed report (Cohen and Sugarman, unpublished). In brief, the initial idea of the study met with three criticisms: offering ART could be coercive due to a lack of availability of ART to the general population in the majority of the countries participating in the study, ART should not be offered at high CD4 cell counts (i.e., above current guidelines), and ART would undermine prevention counseling – the proven intervention – resulting in negative risk compensation behaviors. These concerns are entirely valid. The informed consent process was used to define alternative strategies for potential study subjects to obtain ART at a CD4 cell count consistent with local country guidelines and to describe the potential costs and benefits of earlier ART. Repeated couples counseling during the course of the study was used to reduce increased risk-taking behavior. Indeed, the trial was designed to study the potential benefit of risk-reduction counseling through the measurement of sexual behavior and condom use.
Other concerns arose as the trial was moving forward, including the issue of separation of the role of an investigator vs. their role as a clinical care provider. Because healthcare workers are a rare commodity in countries with a heavy HIV disease bur- den, the participants and caregivers might find it difficult to separate clinical care from research. This problem is sometimes referred to as the `therapeutic misconception' [49]. To mitigate this conflict of interest among the investigators, an `on-call' clinical management team was established, charged with advising all sites on general clinical management and complicated clinical cases within the parameters dictated by the protocol.
Evolving science created yet another challenge for the study team. With increased interest in HIV prevention, a large number of modeling studies [8] and observational studies [50,51,52*,53] purported to resolve both the value of ART for prevention and the `when to start ART' question [54*,55,56,57*]. These reports forced consideration of the equipoise balance required for the trial to proceed [58]. Ultimately, observational treatment studies led to changes in WHO [59] and other guidelines, [60] leading to both practical and ethical concerns about HPTN 052. At the same time, the Swiss AIDS Commission concluded that the benefits of ART had been resolved and offered the `Swiss Statement' to make this point [61]. However, the issues considered (by some) `resolved' by the observational trials were being directly addressed by HPTN 052. The weight of evidence for both early treatment and ART for prevention has been summarized in two Cochrane reviews [62*,63].
Publication of the Comprehensive International Program of Research on AIDS (CIPRA) Haiti study [64*] had additional and important effects on HPTN 052 because of concern that clinical equipoise had been breached, favoring earlier ART. The CIPRA Haiti study was designed to compare early vs. delayed ART. In this study, however, the subjects were enrolled with a mean CD4 cell count of 287 cells/ml, and the delayed arm received ART at a CD4 cell count of 160 cells/ml, which were far different degrees of immunosuppression than studied in HPTN 052.
The results of the CIPRA Haiti study led to an urgent ad-hoc meeting of the Data and Safety Monitoring Board (DSMB) overseeing HPTN 052 in July 2009. The DSMB recommended that HPTN 052 continue without modification to the study design. In November 2009, WHO updated their guidelines for the initiation of ART to at or below a CD4 cell count of 350 cells/ml [59] for the general population. We communicated changes in WHO treatment guidelines to all study participants, along with information about the provision of ART avail- able in their local setting. The study itself underwent no changes, as it was designed specifically to provide `Level 1 evidence' for policy changes predicated on less robust results.
THE EVENTUAL HPTN 052 TRIAL: A SUMMARY
HPTN 052 began in a pilot phase in April 2005 and enrolled 1763 HIV-serodiscordant couples, the vast majority of which (97%) were heterosexual. The study was conducted at 13 sites across Africa, Asia, and the Americas. During a regularly scheduled review in April 2011, the study's DSMB recommended that current results be released to the public as soon as possible. The primary results of HPTN 052 have been published [46**]. For each transmission event, genetic analysis was conducted to determine whether the event was `linked', meaning that the virus had been transmitted between the members of the enrolled couple, or `unlinked', meaning that the new infection had been acquired outside of the primary relationship. Among the 877 couples in the delayed ART group, 27 linked HIV transmissions occurred. This finding was in contrast to only one linked transmission that occurred in the immediate ART group. This difference was highly statistically significant. At least 10 unlinked transmission events also occurred, suggesting that the couples counseling provided through the study was not entirely effective. Clinical benefit was also observed in participants receiving early ART. HPTN 052 provides unambiguous proof that ART can stop the transmission of HIV.
HPTN 052: THE WAY FORWARD
The HPTN 052 study is still ongoing. Given the current results of the trial, participants on the delayed ART arm are being offered ART. With the continuation of the study, we will be able to reliably answer the other important question the study was designed to answer: is the reduction of sexual transmission of HIV by ART durable?
CONCLUSION
HPTN 052 is a complicated and challenging study design to prove a single concept that treating HIV- infected people with effective ART will render them less contagious. This idea is central to the `test and treat' strategy [6]. The results of the study were met with great enthusiasm upon their release in May 2011, and at the 6th International AIDS Society HIV
Pathogenesis Meeting in Rome, July 2011. HPTN 052 represents a nearly 20-year investment in marrying exceptionally strong biological plausibility to a clinical trial. The findings of HPTN 052 were heralded as a `game changer', [65] and became the foundation for The Economist's `The End of AIDS?' issue [66]. The goal now is to determine whether ART can be used so broadly and so effectively as to reduce the spread of HIV within a population. At least two community randomized trials that employ ART as their cornerstone are planned [6], and the results should determine the true benefit of this provocative and very effective intervention.
Table 2A.
HPTN 052 Study Inclusion Criteria
| Couple | Index Case (HIV-infected) | Partner (HIV-uninfected) |
|---|---|---|
|
| ||
|
|
|
Table 2B.
HPTN 052 Study Exclusion Criteria
| Couple | Index Case (HIV-infected) |
|---|---|
|
| |
|
|
Note: There are no explicit exclusion criteria for HlV-uninfected participants (partners)
Acknowledgements
The authors wish to thank the entire HPTN 052 protocol team. Funding was received from NIH via the HIV Prevention Trials Network for all three authors.
Funding Source: NIH HIV Prevention Trials Network (HPTN).
Footnotes
Conflicts of interest No conflicts of interest are declared for any of the authors.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment for the prevention of HIV transmission. J Int AIDS Soc. 2010;13:1–8. doi: 10.1186/1758-2652-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Fidler S. HIV prevention 2010: where are we now and where are we going? Curr Opin HIV AIDS. 2010;5:265–268. doi: 10.1097/COH.0b013e32833acafa. [DOI] [PubMed] [Google Scholar]
- 4.Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment as prevention of HIV transmission: review of scientific evidence and update. Curr Opin HIV AIDS. 2010;5:298–304. doi: 10.1097/COH.0b013e32833a6c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith K, Powers KA, Kashuba AD, Cohen MS. HIV-1 treatment as prevention: the good, the bad, and the challenges. Curr Opin HIV AIDS. 2011;6:315–325. doi: 10.1097/COH.0b013e32834788e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosioni J, Calmy A, Hirschel B. HIV treatment for prevention. J Int AIDS Soc. 2011;14:28–35. doi: 10.1186/1758-2652-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Gay CL. Treatment to prevent transmission of HIV-1. Clin Infect Dis. 2010;50(Suppl 3):S85–S95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153:778–789. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky RP, Paltiel AD, Losina E, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis. 2010;51:392–400. doi: 10.1086/655130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2:487–493. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 12.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 13.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmis- sion: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 14.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by `test and treat' in hyperendemic settings. AIDS. 2010;24:729–735. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Charlebois ED, Das M, Porco TC, Havlir DV. The effect of expanded antiretroviral treatment strategies on the HIV epidemic among men who have sex with men in San Francisco. Clin Infect Dis. 2011;52:1046–1049. doi: 10.1093/cid/cir085. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a modeling exercise specific to men who have sex with men in San Francisco, which concludes that the population would benefit greatly from treatment as prevention.
- 16.van de Vijver D, van Dijk J, Nouwen J, et al. The potential impact of recent infections, HIV testing and start of antiretroviral drugs at a CD4 of <350 on the HIV epidemic in a rural area in Zambia: a mathematical model [abstract #963]. 17th Conference on Retroviruses and Opportunistic Infections (CROI); San Francisco, CA, USA. 16 - 19 February 2010; 2010 ( http://www.retroconference.org/AbstractSearch/Default.aspx?Conf=19) [Google Scholar]
- 17.Heymer KJ, Wilson DP. Treatment for prevention of HIV transmission in a localised epidemic: the case for South Australia. Sex Health. 2011;8:280–294. doi: 10.1071/SH10084. [DOI] [PubMed] [Google Scholar]
- **18.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses clinical and behavioral data from people with acute HIV infection (AHI) to model their effect on the spread of HIV. The results suggest that as many as a third of new cases of HIV can be ascribed to patients with AHI, more than would be anticipated in an established epidemic.
- 19.Baggaley RF, Garnett GP, Ferguson NM. Modelling the impact of antiretroviral use in resource-poor settings. PLoS Med. 2006;3:493–504. doi: 10.1371/journal.pmed.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blower S, Bodine E, Kahn J, McFarland W. The antiretroviral rollout and drug- resistant HIV in Africa: insights from empirical data and theoretical models. AIDS. 2005;19:1–14. doi: 10.1097/00002030-200501030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Law MG, Prestage G, Grulich A, et al. Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS. 2001;15:1287–1294. doi: 10.1097/00002030-200107060-00011. [DOI] [PubMed] [Google Scholar]
- 22.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity follow- ing the introduction of highly active antiretroviral therapy. AIDS. 2004;18:81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190:879–885. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- *24.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:1–9. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use several data sets to argue that the widespread use of ART in San Francisco has reduced the incidence of HIV. The authors used the BED assay to assess incidence that actually failed to show significant differences over time. However, when new diagnoses of HIV were used as a surrogate for HIV incidence, significant reduction was observed. The authors also demonstrate reduced `community viral load' among HIV-infected people in care.
- *25.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use provincial data to demonstrate that the expanded usage of ART correlates with a decrease in new HIV diagnoses. The paper stimulated several letters to the Lancet challenging the methods and conclusions of the work.
- 26.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 27.Henry K, Chinnock BJ, Quinn RP, et al. Concurrent zidovudine levels in semen and serum determined by radioimmunoassay in patients with AIDS or AIDS- related complex. JAMA. 1988;259:3023–3026. [PubMed] [Google Scholar]
- 28.Gilliam BL, Dyer JR, Fiscus SA, et al. Effects of reverse transcriptase inhibitor therapy on the HIV-1 viral burden in semen. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:54–60. doi: 10.1097/00042560-199705010-00009. [DOI] [PubMed] [Google Scholar]
- 29.Gilliam BL. Methods of culturing HIV-1 from semen. Methods Mol Med. 1999;17:51–57. doi: 10.1385/0-89603-369-4:51. [DOI] [PubMed] [Google Scholar]
- 30.Krieger JN, Coombs RW, Collier AC, et al. Recovery of human immunode- ficiency virus type 1 from semen: minimal impact of stage of infection and current antiviral chemotherapy. J Infect Dis. 1991;163:386–388. doi: 10.1093/infdis/163.2.386. [DOI] [PubMed] [Google Scholar]
- 31.Van Voorhis BJ, Martinez A, Mayer K, Anderson DJ. Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification techniques. Fertil Steril. 1991;55:588–594. [PubMed] [Google Scholar]
- 32.Dyer JR, Gilliam BL, Eron JJ, Jr, et al. Quantitation of human immunodeficiency virus type 1 RNA in cell-free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quan- titative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 33.Vernazza PL, Gilliam BL, Flepp M, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997;11:1249–1254. doi: 10.1097/00002030-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Pereira AS, Kashuba AD, Fiscus SA, et al. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis. 1999;180:2039–2043. doi: 10.1086/315149. [DOI] [PubMed] [Google Scholar]
- 35.Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–421. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 36.Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77:4–11. doi: 10.1136/sti.77.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and nonnucleo- side reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J Acquir Immune Defic Syndr. 2004;37:1577–1580. doi: 10.1097/00126334-200412150-00008. [DOI] [PubMed] [Google Scholar]
- 38.Russell JS, Chibo D, Kaye MB, et al. Prevalence of transmitted HIV drug resistance since the availability of highly active antiretroviral therapy. Commun Dis Intell. 2009;33:216–220. [PubMed] [Google Scholar]
- 39.Hurt CB, McCoy SI, Kuruc J, et al. Transmitted antiretroviral drug resistance among acute and recent HIV infections in North Carolina from 1998 to 2007. Antivir Ther. 2009;14:673–678. [PMC free article] [PubMed] [Google Scholar]
- 40.Rahim S, Fredrick LM, da Silva BA, et al. Geographic and temporal trends of transmitted HIV-1 drug resistance among antiretroviral-naive subjects screen- ing for two clinical trials in North America and Western Europe. HIV Clin Trials. 2009;10:94–103. doi: 10.1310/hct1002-94. [DOI] [PubMed] [Google Scholar]
- 41.Bartolo I, Rocha C, Bartolomeu J, et al. Antiretroviral drug resistance surveil- lance among treatment-naive human immunodeficiency virus type 1-infected individuals in Angola: evidence for low level of transmitted drug resistance. Antimicrob Agents Chemother. 2009;53:3156–3158. doi: 10.1128/AAC.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 43.Kalichman SC, Cage M, Barnett T, et al. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS Res Hum Retroviruses. 2001;17:1695–1703. doi: 10.1089/08892220152741397. [DOI] [PubMed] [Google Scholar]
- 44.Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 45.Vettore MV, Schechter M, Melo MF, et al. Genital HIV-1 viral load is correlated with blood plasma HIV-1 viral load in Brazilian women and is reduced by antiretroviral therapy. J Infect. 2006;52:290–293. doi: 10.1016/j.jinf.2005.06.002. [DOI] [PubMed] [Google Scholar]
- **46.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]; These are the primary results for the study discussed in this article. The study randomized patients with a median CD4 cell count of 446 cells/ml to either immediate or delayed ART. Immediate ART reduced HIV transmission from an HIV-infected individual to their HIV-negative partner by >96%, and early ART provided modest clinical benefit to the HIV-infected individual as well.
- 47.Safren SA, Hendriksen ES, Smeaton L, et al. Quality of life among individuals with HIV starting antiretroviral therapy in diverse resource-limited areas of the world. [Accessed 16 April 2001];AIDS Behav. 2001 doi: 10.1007/s10461-011-9947-5. http://www.springerlink.com/content/at42l5rx7hk 57516/fulltext.pdf. [DOI] [PMC free article] [PubMed]
- 48.Firnhaber C, Smeaton L, Saukila N, et al. Comparisons of anemia, thrombo- cytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the America. Int J Infect Dis. 2010;14:e1088–e1092. doi: 10.1016/j.ijid.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson GE, Churchill LR, Davis AM, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS Med. 2007;4:1735–1738. doi: 10.1371/journal.pmed.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunnell R, Ekwaru JP, Solberg P, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan P, Kayitenkore K, Chomba E, et al. Reduction of HIV transmission risk and high risk sex while prescribed ART: results from discordant couples in Rwanda and Zambia [abstract #52bLB]. 16th conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 8 - 11 February 2009; 2009 ( http://www.retroconference.org/AbstractSearch/Default.aspx?Conf=18) [Google Scholar]
- *52.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using data from a randomized, controlled trial, the authors relate ART use to falling CD4 cell count (outside the randomized controlled trial) to demonstrate reduced probability of HIV transmission. The number of person years of follow-up was limited. Transmission probability was greater at lower CD4 cell counts.
- 53.Del Romero J, Castilla J, Hernando V, et al. Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ. 2010;340:1–8. doi: 10.1136/bmj.c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an observational study of more than 20 000 participants in the USA and Europe. The results suggest some modest benefit from earlier ART (>500 CD4 cell count) in AIDS-free survival, but no significant difference in mortality relative to ART started between 300 and 500 cells/ml.
- 55.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 56.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Writing Committee for the CASCADE Collaboration Timing of HAART Initiation and Clinical Outcomes in Human Immunodeficiency Virus Type 1 Seroconverters. Arch Intern Med. 2011;171:1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]; Between 1996 and 2009, this study generated 50 000 person years of follow-up with 9455 patients in Europe. Starting therapy before CD4 fell below 500 cells/ml (but not between 500 and 799 cells/ml) reduced HIV disease progression.
- 58.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. [PubMed]
- 60.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 61.Vernazza P, Hirschel P, Bernasconi E, Flepp M. HIV-positive individuals without additional sexually transmitted diseases (STD) and on effective antiretroviral therapy are sexually non infectious (Les personnes seropositives ne souff rant d'aucune autre MST et suivant un traitment antiretroviral effi cie ne transmettent pas le VIH par voie sexuelle) Bull Med Suisses. 2008;89:165–169. [Google Scholar]
- *62.Anglemyer A, Rutherford GW, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Data- base Syst Rev. 2011:CD009153. doi: 10.1002/14651858.CD009153. [DOI] [PubMed] [Google Scholar]; This review summarizes all data that support `treatment as prevention'.
- 63.Siegfried N, Uthman OA, Rutherford GW. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Database Syst Rev. 2010:CD008272. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors enrolled patients at a median CD4 count of 287 cells/ml to either immediate or delayed ART. Patients in the delayed ART arm (median 160 cells/ml) had significant morbidity and mortality.
- 65. [Accessed 27 September 2011];UNAIDS Executive Director Michel Sidibe' gives guest lecture at Vatican International Study Meeting on HIV. 2011 http://www.unaids.org/en/resources/presscentre/featurestories/2011/may/20110530vatican.
- 66.AIDS: The 30 years war. The Economist. 2011 Jun 4; [Google Scholar]


