Abstract
Stem cell niches, the discrete microenvironments in which the stem cells reside, play a dominant part in regulating stem cell activity and behaviours. Recent studies suggest that committed stem cell progeny become indispensable components of the niche in a wide range of stem cell systems. These unexpected niche inhabitants provide versatile feedback signals to their stem cell parents. Together with other heterologous cell types that constitute the niche, they contribute to the dynamics of the microenvironment. As progeny are often located in close proximity to stem cell niches, similar feedback regulations may be the underlying principles shared by different stem cell systems.
Adult stem cells are undifferentiated and long-lived cells, which are found in most tissues throughout the human body (FIG. 1). They have the remarkable capacity to replenish themselves through self-renewal and to give rise to either one (unipotent) or more (multipotent) downstream differentiated cell lineages.
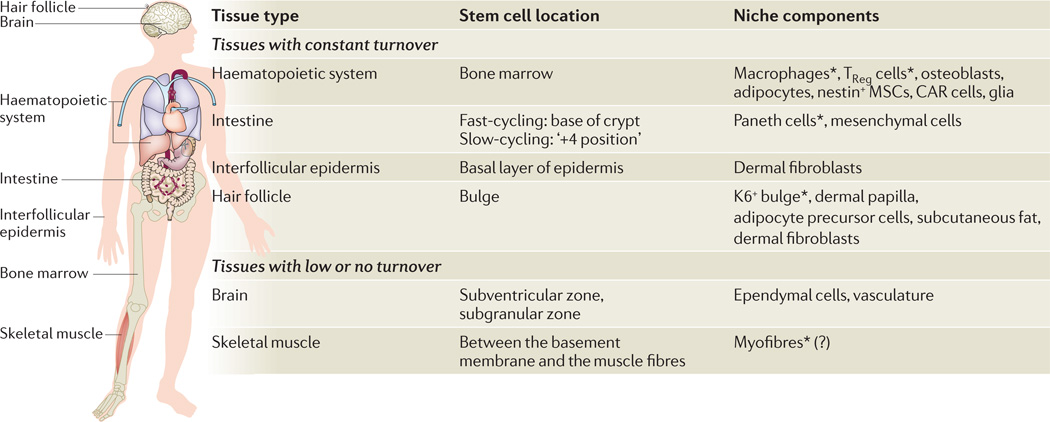
Figure 1. Overview of adult stem cells.
Adult stem cells are undifferentiated cells found in many organs throughout the human body. They are capable of both long-term self-renewal and generation of the downstream differentiated cells of an organ. They can be categorized into two major groups: those with high turnover and those with low turnover. In tissues with high turnover rates, such as the haematopoietic system, intestine, interfollicular epidermis and hair follicle, stem cells are responsible for maintaining homeostasis and also for repairing damage upon wounding. Stem cells have also been reported in tissues with low or no turnover, such as the brain and the skeletal muscle. In the skeletal muscle, stem cells are mainly reserved for repair after injury. In the brain, stem cells are required for the generation of defined subsets of neurons that migrate to the olfactory bulb (stem cells in the subventricular zone) and for the generation of new neurons upon learning stimulations (stem cells in the subgranular zone). The locations of a number of stem cells and their reported niche components are listed in the table. Niche components derived from stem cells are denoted by asterisks. The question mark denotes a candidate niche component whose precise regulatory role remains to be demonstrated. CAR cells, CXCL12-abundant reticular cells; K6, keratin 6; MSCs, mesenchymal stem cells; TReg cells, regulatory T cells.
Epidermal, blood and intestinal cells undergo daily turnover as part of their normal differentiation process, necessitating constant use of stem cells. In the hair follicle, stem cells are needed only periodically to fuel cyclical bouts of hair growth1–3. However, even within relatively dormant tissues, such as adult skeletal muscle and brain, cell-type specific stem cells still exist4–7. Such stem cells undergo extremely low or no division during normal homeostasis but can respond efficiently to stimuli, such as learning activities in the brain or injury in the muscle.
In the skeletal muscle, stem cells are sandwiched between mature muscle fibres and the basement membrane. Upon injury, they proliferate and differentiate to form new myofibres8. In the central nervous system, stem cells are found in the subventricular zone, where they generate neurons that migrate to the olfactory bulb6, and in the subgranular zone of the hippocampus, where adult neurogenesis has been linked to learning and memory7,9.
In most tissues, stem cells are used sparingly, leaving the bulk of tissue regeneration to transiently dividing but committed progeny. As such, the stem cells often remain quiescent for extended periods of time and are only called into action briefly upon initiation of homeostasis or injury. In a few cases, such as in the epidermis and intestine, stem cells seem to be in a state of perpetual action, with the epithelium producing differentiating cells daily10,11.
In malignant tissues, cancer stem cells appear to hijack mechanisms used to regulate normal stem cell functions. Like many of their normal counterparts, cancer stem cells can exist in both slow-cycling and more-rapidly cycling states12–15. Thus, to appreciate how stem cell behaviour changes in pathological states (injury, degeneration and cancer), it is crucial to understand stem cell behaviour under physiological (development and homeostasis) conditions.
Stem cells reside in specific anatomical locations known as niches, which create unique microenvironments (BOX 1). Each niche has a dominant role in controlling stem cell behaviour. Signals from the niche influence the cycling status of stem cells and also help to maintain their undifferentiated state. The existence of the niche was initially postulated by Schofield16, but it remained an abstract concept until niche cells for Drosophila melanogaster female and male germline stem cells were identified and characterized17–19. With recent advances in niche component isolation methods, imaging techniques and genetic tools, various mammalian stem cell niches have been identified with increasing precision3,20–27. With these tools, many intriguing regulatory mechanisms have begun to unfold.
Box 1 | Stem cell niche: past and present.
The term “stem cell niche” was coined by Schofield16. Infused bone marrow cells were known to generate clones of maturing haematopoietic cells in the spleens of irradiated mice, but the efficiency varied significantly with cytotoxic regents. To explain this, Schofield proposed that stem cells associate with other cells to specify their behaviours. Unless transplanted bone marrow cells occupied a similar stem cell niche to their native location, they drifted towards differentiation. Long before a molecular understanding existed, the stem cell niche concept received support from bone marrow transplants used to treat leukaemia: successful transplantations required the irradiation of existing haematopoietic stem cells (HSCs) within the endogenous bone marrow of a patient, suggesting that the transplanted and endogenous HSCs might compete for a limited source of supporting niche cells.
The first stem cell niche described in molecular terms was that of the Drosophila melanogaster female germline stem cells (GSCs). Lineage-tracing studies and laser ablation experiments located GSCs at the anterior tip of ovary germarium113,114. More than two decades after Schofield’s initial hypothesis, an adjacent cluster of postmitotic somatic cells, the ‘cap cells’, were discovered as a source of the bone morphogenic protein (BMP) homologue Decapentaplegic (DPP), which is required for GSC self-renewal and maintenance115,116. When resident GSCs were ablated, differentiating daughters entered the vacated space and made contact with the cap cells, prompting them to de-differentiate and become GSCs17. This differs from the mammalian hair follicle and intestine, where fully differentiated progeny can reside in the niche microenvironment without reversion46,83. In this regard, each stem cell lineage seems to be tailored for its own ‘point of no return’.
Traditionally, the stem cell niche has been viewed as a microenvironment that maintains stem cells and promotes their self-renewal. With the complex and diverse regulations emerging in recent years, it is perhaps now more precise to describe the niche as a local tissue microenvironment that hosts and influences the behaviours or characteristics of stem cells. It is becoming increasingly clear that niche cells can enhance dormancy, promote activation transiently or maintain stem cells in their undifferentiated state — and all of these features are essential to preserve stem cell functions in the long term.
Traditionally, niche cells were believed to be derived exclusively from cell lineages different from those of the stem cells they regulate. Studies from both vertebrate and invertebrate niches have now begun to change this view, identifying stem cell progeny as critical components that impart feedback to their powerful parents to control stem cell activity and behaviour. In this Review, we summarize recent progress in delineating various types of feedback mechanisms provided by stem cell progeny, and we discuss emerging similarities and differences between systems. Although this research area is still in its infancy, the examples to date favour the notion that feedback signalling from progeny to parent may be an important and common strategy to control stem cell activity during homeostasis, wound repair and cancer.
D. melanogaster male germline
The D. melanogaster male germline was one of the first established models for studying interactions between stem cells and their niche.
Structure of the ‘hub’
The D. melanogaster testis is an elongated tubular structure that is closed at the apical end and connected to the rest of the genital tracks at the basal side. The ‘hub’, which is composed of approximately 10–15 somatic cell clusters, resides at the apical end. Germline stem cells (GSCs) and somatic cyst stem cells (CySCs) connect to the hub through adherens junctions. Approximately 8–10 GSCs and twice as many CySCs encase the hub28 (FIG. 2a).
Figure 2. CySCs contribute to ‘hub’ maintenance.
a | Structure of the Drosophila melanogaster testis. The D. melanogaster male germline stem cell niche, located at the apical tip of the testis, hosts two kinds of stem cells: the germline stem cells (GSCs) and the somatic cyst stem cells (CySCs). The ‘hub’ cells at the apical tip are critical niche components. GSCs produce gonialblasts, which undergo synchronous divisions to generate spermatogonia and eventually mature sperm. CySCs generate somatic cyst cells, which surround the gonialblasts and maturing spermatogonia. b | A crucial signalling molecule from the hub is Unpaired (UPD), which activates the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway in adjacent CySCs to promote their self-renewal by activating transcription factors ZFH1 (zinc-finger homeodomain 1) and CHINMO (chronologically inappropriate morphogenesis). In GSCs, the JAK–STAT pathway functions to facilitate their attachment to the hub via adherens junctions. In addition, the Bone morphogenic protein (BMP)–like molecules Decapentaplegic (DPP) and Glass bottom boat (GBB) are secreted from both the hub and the CySCs to activate BMP signalling in GSCs and repress the differentiation factor bag of marbles (bam). Together, this permits GSC self-renewal. c | In some cases, CySCs also generate cells that can contribute to the hub.
GSCs divide asymmetrically to produce one daughter cell that maintains stem cell identity and another, the gonialblast, that initiates a differentiation programme. Gonialblasts divide synchronously with incomplete cytokinesis to form 16 interconnected germ cell cysts that will terminally differentiate and become mature sperm. CySCs also divide asymmetrically, producing cyst cells that enclose the differentiating germ cells. It is thought that cyst cells provide essential signals to promote germ cell differentiation through Epidermal growth factor (EGF) signalling29.
Signalling events in the hub
The hub has long been established as a critical niche component for both types of stem cells. The hub secretes an interleukin-like cytokine called Unpaired (UPD), which activates Janus kinase (JAK) signalling in both GSCs and CySCs. The activated downstream effector, phosphorylated Signal transducer and activator of transcription (STAT), is necessary and sufficient for CySC self-renewal18,19. Two downstream targets of JAK–STAT were identified in CySCs: the zinc-finger transcription repressor zfh1 (zinc-finger homeodomain 1)30 and the BTB (BR-C, TTK and BAB) domain-containing nuclear factor chinmo (chronologically inappropriate morphogenesis)31. Both are necessary and sufficient to promote self-renewal of CySCs and to maintain their undifferentiated status. By contrast, JAK–STAT activation in GSCs is required for their anchorage to the hub but not for their self-renewal. Instead, GSC self-renewal relies on sustained signalling through Bone morphogenic protein (BMP)-like ligands Decapentaplegic (DPP) and Glass bottom boat (GBB), which are secreted from both the hub and the CySCs32–34. The outcome is transcriptional repression of a differentiation factor, bag of marbles (bam), resulting in the suppression of GSC differentiation into gonialblasts34 (FIG. 2b). The interactions between GSCs and CySCs and their interdependencies are intriguing paradigms for studying the coordinated behaviours of multiple stem cell types within a shared niche.
CySCs and the hub: more alike than different
It was assumed that, when fully developed, the hub remains static and permanent. However, recent experiments suggest that, under some circumstances, CySCs might contribute to the mature hub35. The rationale behind this work was simple: if cells outside the hub are marked by either bromo-deoxyuridine (BrdU) or transgenes, then, following a chase period, markers will be observed within the hub only if its residents are dynamic rather than static. To test this possibility, researchers exploited the fact that hub cells are terminally differentiated and therefore do not divide. When β-galactosidase-expressing (βGAL+) CySC clones were generated through mitotic recombination36, βGAL+ hub cells were initially absent but appeared following a chase period. Similarly, upon administration of a BrdU pulse, BrdU-positive hub cells were detected within several days. Thus, CySCs not only make the somatic cyst cells but also can contribute to their niche by making hub cells (FIG. 2c). Although CySC contribution to the hub might be rare during steady state, when CySCs carry mutations in the lines gene, they transform their fate to adopt characteristics of hub cells. Interestingly, lineage-tracing experiments further suggest that hub cells and CySCs originate from a common precursor pool during embryogenesis37. Together, these data point to a close relationship between CySCs and hub. In the future, it will be interesting to see whether CySCs increase their contribution to hub cells during ageing or stress.
The hair follicle and its stem cells
The mouse hair follicle has emerged as an important paradigm in which to study stem cells and their niche.
Structure of the bulge: a shared stem cell niche
Epithelial hair follicle stem cells (HFSCs) reside in an anatomical region known as the bulge, which is located below the sebaceous glands at the base of the non-cycling portion of follicles. Slow-cycling HFSCs are located in the outermost layer and are marked by high levels of integrins (α6β4 and α3β1), keratins (keratin 5 (K5), K14 and K15) and cell surface markers (CD34 and Leu-rich repeat-containing G protein-coupled receptor 5 (LGR5))3,38,39. They also express transcription factors, including SOX9, LIM homeobox 2 (LHX2), nuclear factor of activated T cells, cytoplasmic 1 (NFATC1), T cell factor 3 (TCF3) and TCF4. Loss-of-function studies show that all of these factors help to maintain features of ‘stemness’ (REFS 40–45).
The inner layer of bulge cells forms at the end of the hair cycle (see below). These cells do not express integrins or CD34 but they do express some HFSC markers, including NFATC1, SOX9, LHX2 and TCF3. They also exhibit features not seen in HFSCs, including unusual intercellular adhesions to the hair shaft and expression of K6 (REF. 46).
The bulge base features a small cluster of cells, known as the hair germ. Transcriptional profiling shows that hair germ cells are more similar to HFSCs than to their transient amplifying progeny, that is, to matrix cells (see below)47. Except for NFATC1 and CD34, the hair germ expresses the HFSC markers mentioned above. They can be distinguished from the bulge HFSCs by their high levels of placental cadherin (P-cadherin).
The bulge niche hosts not only HFSCs but also melanocyte stem cells (MCSCs), which are interspersed between HFSCs48. The behaviours of HFSCs and MCSCs are coordinated, enabling differentiating MCSCs to generate and transfer pigment to matrix cells as they begin to terminally differentiate to produce hair shaft cells49,50. The bulge niche has also been implicated as a residence for smooth muscle progenitors responsible for generating the arrector pili muscle, which is located just above the bulge51.
Components of the HFSC niche
Why do so many stem cells remain in and near the bulge? One reason is that the bulge offers a rich milieu for various regulatory inputs coming from a diverse array of cell types. Converging on the upper bulge are four different types of sensory neurons, providing a means of coordinating sensory responses in the skin with stem cell behaviour52. Additionally, the bulge is encased by blood vessels, providing hormonal and nutrient inputs to stem cells.
Much of what we know about the other components of the niche and their signalling is focused on their relation to HFSC regulation. HFSCs themselves express several secreted factors that regulate their own behaviour39,53. Additionally, several different cell types in and around the bulge niche are likely to participate in regulating HFSC activity (FIG. 3a). Within the niche, MCSCs are immediate HFSC neighbours and likely candidates for crosstalk50. In the inner bulge layer, K6+ cells are particularly interesting, in that they are progeny of HFSCs and function in maintaining HFSC quiescence46. On the other side of the extracellular matrix-rich basement membrane, which separates dermis from epithelium, are counteracting signals that promote stem cell activation and proliferation. A major source of these signals is the dermal papilla, which is located just beneath the hair germ and has long been established as an essential activation centre54–56. The HFSC and hair germ niche is surrounded by a sheath of dermal fibroblasts, which can also provide local activating or inhibitory factors for HFSCs57.
Figure 3. The HFSC niche: a dynamic interplay between HFSC progeny and dermal components.
a | Hair follicle stem cells (HFSCs) reside in the outer bulge layer. Keratin 6-expressing (K6+) inner bulge cells are downstream progeny of HFSCs and express high levels of bone morphogenic protein 6 (BMP6) and fibroblast growth factor 18 (FGF18), which are quiescence-inducing factors for HFSCs. During telogen, subcutaneous adipocytes express BMP2 and dermal fibroblasts express BMP4. Near the end of the resting phase, the dermal papilla produces HFSC-activating factors, including FGF7 and FGF10, BMP inhibitors and transforming growth factor-β2 (TGFβ2) to counteract the inhibitory effects on the niche. TMEFF1 (transmembrane protein with EGF-like and two follistatin-like domains 1) is induced in hair germ cells by TGFβ2 to dampen the suppressive effects of BMPs. In addition, adipocyte precursor cells secrete platelet-derived growth factor-α (PDGFα) to induce the expression of as-yet-unidentified activating factors in the dermal papilla. The macroenviroment of the underlying dermis also participates, progressing to a BMP-low and WNT-high state. b | Once activating signals accumulate over a threshold level, HFSCs at the base of the bulge (the hair germ), begin to proliferate to initiate hair growth (anagen). The dermal papilla is pushed away from the bulge, and HFSCs return to quiescence. Two populations of outer root sheath (ORS) cells are spared at catagen and form a new bulge at telogen: the slow-cycling ORS cells close to the old bulge form the new outer bulge layer, and the faster cycling ORS cells nearer to the matrix differentiate and become the new inner K6+ bulge layer.
Two longer range signalling inputs participate in regulating HFSC behaviours. Subcutaneous adipocytes send out waves of alternating inhibitory and activating factors that help synchronize HFSC niches within the skin57. Most recently, adipocyte precursors were implicated in transmitting regulatory factors that probably signal to receptors on the surface of the dermal papilla and/ or dermal sheath58. Although we currently understand only a fraction of the extensive crosstalk within these many cell types, the intriguing picture emerging is that multiple niche components regulate stem cell behaviour at different hair cycle stages (see the discussion below on specific cell types and signals involved to drive or inhibit hair growth). It seems likely that many of these cells will also harbour regulatory functions towards other stem cell residents of the bulge.
Activation of HFSCs by dermal niche components
Hair follicles undergo cyclical bouts of growth (anagen), destruction (catagen) and rest (telogen), which are collectively known as the hair cycle59. This unique feature has provided insights into the general process of tissue regeneration during homeostasis.
HFSCs remain quiescent throughout most of the hair cycle2,3. At the start of the hair growth phase, HFSCs become activated briefly47,60. Stem cell activation is complex, and the factors involved are still being discovered. Two critical pathways are activation of WNT signalling and inhibition of the BMP pathway. Together, these stabilize nuclear β-catenin in the hair germ and promote hair follicle regeneration47,61–69.
During the quiescent phase of the hair cycle, hair germ cells produce increasing levels of WNTs while the dermal papilla produces increasing levels of BMP inhibitory factors47,56. At the telogen–anagen transition, transforming growth factor-β2 (TGFβ2) is produced and transmitted by the dermal papilla. Hair germ cells respond by inducing TMEFF1 (transmembrane protein with EGF-like and two follistatin-like domains 1), which inhibits the BMP pathway, thereby activating HFSCs70. In addition, intradermal adipocyte precursors secrete platelet-derived growth factor-α (PDGFα), which further stimulates the mesenchyme surrounding the bulge58. Together, these accumulating activation forces transmitted to HFSCs finally overcome strong inhibitory thresholds from other cells within the niche, thereby triggering a new hair cycle. When this happens, the hair germ begins to proliferate and reform the new hair follicle, followed by HFSC proliferation in the bulge 1–2 days later.
HFSCs divide several times as they exit the bulge, forming a downward trail that extends from the bulge along the exterior (the outer root sheath (ORS)) of the regenerating hair follicle. Meanwhile, the dermal papilla is pushed further downward, away from the bulge. As the dermal papilla becomes more distant, bulge and upper ORS cells return to quiescence. Lower ORS cells become increasingly more proliferative as they approach the dermal papilla and change into transient amplifying matrix cells.
In the mature hair follicle, matrix cells envelope the dermal papilla, forming a bulb-like structure at the base. There, transient amplifying matrix cells divide rapidly but transiently before terminally differentiating to produce the new hair and its channel71. The dynamic movement of the dermal papilla from the bulge to the transient amplifying matrix during the hair cycle may contribute to the prompt return of the HFSCs to quiescence and result in a distinct pool of highly proliferative transient amplifying cells to fuel this tissue-producing factory.
Interactions that resemble those of stem cells in the D. melanogaster testis were reported recently as occurring between MCSCs and HFSCs. During anagen onset, WNT signals activated at the bulge base promote MCSC proliferation and differentiation50. As activated MCSCs move downwards with the HFSC progeny, they generate pigment, transferring it to the transient amplifying precursors of the hair shaft. This collaboration between HFSCs and MCSCs within the hair follicle offers a valuable mammalian model to probe the interactions and coordination of multiple stem cell types within the same niche.
Feedback signals from progeny switch HFSCs off
Upon exhausting their proliferative potential, the matrix population undergoes apoptosis, triggering catagen that stops hair growth. During this time, most cells within the lower ORS also undergo apoptosis, as the remaining epithelial strand and basement membrane retracts upward, bringing the dermal papilla with it. HFSCs in the upper portion of the ORS are spared and become the new bulge for the next cycle. HFSCs that progressed a bit further along the ORS become the hair germ.
Surprisingly, a few committed cells in the lower ORS also survive catagen. With matrix cells undergoing apoptosis, these ORS cells bypass the transient amplifying step, undergoing an atypical differentiation route and settling in the inner K6+ bulge layer (FIG. 3b). Despite retaining many stem cell markers, K6+ cells do not function as stem cells during homeostasis or upon wounding. Rather, K6+ bulge cells form specialized intercellular junctions that anchor the old hair shaft and prevent the animal from losing all its hairs at the end of the hair cycle. In addition, these cells produce high levels of fibroblast growth factor 18 (FGF18) and BMP6 (REF. 46), which are quiescence factors that are also expressed at low levels by HFSCs39. Notably, if K6+ bulge cells are either removed through hair plucking or ablated through preferential expression of diphtheria toxin receptor (DTR) followed by diphtheria toxin administration, bulge HFSCs become prematurely activated and enter a new round of hair growth. Importantly, injections of BMP6 or FGF18 prevent this early activation, suggesting that the K6+ bulge restricts HFSC activity in part through these factors. This discovery is an example in which stem cell progeny can act as a key signalling centre to stem cells and maintain their quiescence in the niche46.
Committed MCSC lineage cells are not known to return back to the bulge at the end of catagen. However, repeated hair plucking, which depletes K6+ bulge cells, also results in early hair greying. This could be a sign of MCSC exhaustion owing to overactivation72. In the future, it will be interesting to probe deeper into the possible crosstalk between K6+ bulge cells and both HFSCs and MCSCs.
Joint efforts of microenvironment and macroenvironment
Once hair follicles re-enter the quiescent phase, both HFSCs and MCSCs can remain dormant for months. During this time, the stimulatory dermal papilla abuts against the HFSCs, hair germ and MCSCs, necessitating strong inhibitory signals from the surrounding environment to maintain stem cell quiescence. In addition to K6+ bulge cells, the global dermal macroenvironment, including fibroblasts and subcutaneous adipocytes, secretes BMP2 and BMP4 and contributes to the BMP-high, WNT-low state of the niche at this time. This status progressively changes so that, by the end of telogen, dermal BMP levels decline while WNTs rise57,68. Thus, together with local crosstalk between stem cells and neighbouring niche components, long-range signals from the macroenvironment shift the BMP-high and WNT-low state to a BMP-low and WNT-high one, thereby favouring the telogen to anagen transition. These macroenvironmental cues reinforce synchronized stem cell behaviour across the animal’s coat.
In summary, the hair follicle system is an example of how stem cell progeny and heterologous niche cell types either counterbalance each other’s effects or cooperate to ensure proper stem cell activity during homeostasis. Moreover, the ability of HFSC progeny to return to the niche and impose regulatory signals to stem cells may provide a paradigm for stem cell niches, which must orchestrate when to make tissue and when to stop. The communication network in this busy corner of the hair follicle appears to be handling quite a cacophony of signalling circuits, and it remains a wonder that the ‘wires’ do not get crossed.
The mouse intestine
The small intestinal epithelium is among the most rapidly self-renewing tissues in mammals. The entire intestinal epithelium turns over in 3–5 days in a constant effort to fuel the nutritional uptake that maintains the body. This rapid homeostasis is sustained by intestinal stem cells (ISCs).
Coexistence of slow-cycling and fast-cycling ISCs
The G protein-coupled receptor LGR5, is expressed at the bottom of the crypt. A tamoxifen-inducible Cre–oestrogen receptor (Cre–ER) recombinase under control of the Lgr5 promoter was used to excise a floxed stop codon that otherwise prevents a genetically manipulated Rosa26–LacZ locus from being ubiquitously expressed. After Cre–ER activation, βGAL was detected in LGR5+ cells and, soon after, in all of their progeny. All cell types within crypts and villi were marked in a long-term manner, suggesting that LGR5+ cells are bona fide ISCs and not a transient amplifying population25. Despite proliferative differences between fast-cycling LGR5+ ISCs and slow-cycling HFSCs, they share many key stem cell genes, including Lgr5, Sox9 and Tcf4. As seen in hair follicles, BMP signalling imposes a quiescence force, whereas WNT signalling is crucial for ISC activation73,74.
Historically, a population of slow-cycling cells (BOX 2) were identified around the fourth position from the bottom of the crypt (‘position +4’), above the LGR5+ cells75. Several proteins mark these slow-cycling cells, such as Polycomb complex subunit BMI1 (REF. 76), telomerase reverse transcriptase (TERT)77 and the atypical homeodomain-containing protein HOPX1 (REF. 78). Interestingly, analogous lineage-tracing experiments using Bmi1–Cre–ER, Tert–Cre–ER or Hopx1–Cre–ER to mark the +4 cells have provided compelling evidence that these cells can also generate all cell types within crypts and villi, including the LGR5+ cells. Importantly, when the LGR5+ cells were ablated, these slow-cycling ISCs expanded in number and efficiently reconstituted the LGR5+ population79. This apparent functional equivalency and lack of hierarchy suggests that, like the stem cells of the hair follicle and bone marrow, ISCs can transition between slow-cycling and fast-cycling states. In the future, it will be important to determine the relative contributions of these states to tissue formation during homeostasis and upon injury. Given the rapid turnover requirements of the intestinal epithelium, it seems probable that fast-cycling ISCs may contribute more to homeostasis, whereas slow-cycling ISCs are reserved mainly for injury conditions, such as ionizing radiation. A similar situation has previously been described for the haematopoietic system26,80.
Box 2 | Label-retaining assay: facts and myths.
A label-retaining assay contains two essential parts: a pulse period and a chase period. Historically, bromo-deoxyuridine (BrdU) or radiolabelled DNA analogues were administrated into the animals for a certain time (the pulse) to label all of the proliferating cells. The labelling reagents were then taken away for a prolonged period (the chase) before the tissues were examined. Fast-cycling cells are constantly dividing and dilute the label through each round of division. Therefore, after the chase, their original label is diluted to a degree in which it can no longer be detected. Conversely, slow-cycling cells divide infrequently during the chase period. Therefore, they retain significant amounts of the label and appear as label-retaining cells (LRCs). Recently, a system combining two transgenes, a fluorescent histone (histone H2B labelled with green fluorescent protein (H2B–GFP)) under the control of tetracycline (TET) response element (TRE) and a tissue-specific promoter driving a TET repressor, has been successfully applied to label-retaining assays. When the transgenic animals are fed food containing doxycycline (the chase), synthesis of new H2B–GFP is suppressed, and only infrequently dividing cells retain the H2B–GFP label3.
Label-retaining assays provide a powerful way to delineate the cycling properties within a given population. However, caution must be taken when designing and interpreting the experimental results. First, although many stem cells are slow-cycling, label retention on its own does not imply ‘stemness’. In fact, cells that divide during the pulse period and soon withdraw from the cell cycle and differentiate will also appear as LRCs46. Moreover, cells with extremely low proliferation kinetics but which do not proliferate during the pulse window will not incorporate the label and hence will be overlooked despite their slow-cycling features. To correctly infer the cycling status of a given cell, label-retaining assays must be combined with careful analysis of cell cycle kinetics. Finally, to demonstrate that a cell is a stem cell, rigorous lineage-tracing assays need to be performed to document long-term tissue-regenerative capacity.
Paneth cells regulate the LGR5+ ISCs
Which cells are the niche cells for ISCs? Neighbouring mesenchymal cells were initially proposed to be crucial ISC niche stimulants but, thus far, in situ hybridization experiments have failed to map specific ISC stimuli to these cells81. By contrast, recent studies have brought Paneth cells, which are interspersed between the LGR5+ ISCs, to the forefront of the candidate list. Paneth cells secrete antimicrobial molecules and have long been known for their defensive roles82. Lineage-tracing analyses confirmed that Paneth cells are produced directly by ISCs and, in this regard, they constitute ISC progeny that are located at the crypt niche25.
The relationship between the Paneth cells and the ISC niche has long been postulated. However, it was only recently that researchers demonstrated that Paneth cells might function in ISC maintenance83,84. The first evidence that Paneth cells may be critical ISC niche cells came from in vitro studies. By combining Paneth and LGR5+ cells in culture, the efficiency of forming organoids that resemble the gut was increased significantly relative to that of LGR5+ cells alone83. To show that Paneth cells function similarly in vivo, three different mouse models known to affect Paneth cell numbers were used83: a mutation in the zinc-finger protein growth factor-independent 1 (Gfi1)85; a conditional knockout of Sox9 (REFS 86,87); and expression of diphtheria toxin under a Paneth cell-specific promoter88. When Paneth cell numbers were reduced, the numbers of ISCs were also reduced accordingly (FIG. 4). Interestingly, the remaining ISCs clustered around the surviving Paneth cells83.
Figure 4. ISCs generate Paneth cells that promote stem cell proliferation at the base of the intestinal crypt.
Lineage-tracing experiments suggest that the Leu-rich repeat-containing G protein-coupled receptor 5-expressing (LGR5+) intestinal stem cells (ISCs; shown in green) at the bottom of the crypt generate all of the differentiated lineages of the intestinal epithelium, including their own niche cells — the Paneth cells (shown in yellow). Paneth cells maintain ISCs both in vitro and in vivo. When Paneth cell numbers are reduced owing to mutations or ablations, the numbers of LGR5+ ISCs are also reduced accordingly, and the remaining ISCs cluster around the leftover ISCs. WNT3, epidermal growth factor (EGF), transforming growth factor-α (TGFα) and Delta-like ligand 4 (DLL4) are all factors expressed by the Paneth cells and known to influence the maintenance of ISCs either in vivo or in vitro. A slow-cycling population of ISCs, commonly referred to as the ‘+4 ISCs’ owing to their position fourth from the bottom of the crypt (shown in purple), are located at the periphery of the Paneth cell-rich zone and nearest to the bone morphogenic protein (BMP)-expressing mesenchymal cells. Several genes have been identified to be expressed at these cells, including those encoding Polycomb complex subunit BMI1, telomerase reverse transcriptase (TERT) and atypical homeodomain-containing protein HOPX1. Their unique location might also contribute to their slow-cycling status.
It is not yet clear whether Paneth cells act by secreting paracrine factors that sustain ISC survival and/or proliferation or whether they function to suppress ISC differentiation. Transcriptional profiling has revealed that Paneth cells express Wnt3, Egf, Tgfa and Delta-like ligand 4 (Dll4), which, at least in culture, are all factors that are important for ISC proliferation and maintenance81,89,90. Among the pathways controlled by these factors, the canonical WNT pathway appears to be the primary signal that promotes intestinal epithelial maintenance, as evidenced by Tcf4 loss-of-function mutations73 and by recent molecular and genetic links established between WNT receptors and the ISC G protein-coupled receptors LGR5 and LGR4 (REF. 91). The LGR ligands are roof plate-specific spondins (R-spondins), which are known to stimulate WNT–β-catenin signalling84,92. Future studies should aim to demonstrate the in vivo relevance of these myriad Paneth-secreting factors and to firmly link these candidate factors to the importance of Paneth cells as key LGR5+ ISC niche components. Depletion of candidate factors specifically in the Paneth cells might help to advance this area. However, the capacity of Paneth cells to stimulate the proliferation of ISCs seen in vitro suggests that Paneth cells can no longer be viewed merely as immune surveillance guardians of ISCs.
Although Paneth cells might facilitate ISC self-renewal, this does not exclude the importance of underlying mesenchymal cells. Mesenchymal cells at the crypt–villus border express BMP2 and BMP4, which may halt proliferation and promote differentiation of ISCs at this juncture74,93. Other signals from mesenchymal cells, such as the secreted Frizzled-related proteins (sFRPs), might also attenuate and modulate WNT signalling to prevent over-proliferation of ISCs81.
Comparisons between the HFSC niche and the ISC niche
When taken together, several interesting commonalities emerge between HFSCs and ISCs. Both are influenced similarly by WNTs and BMPs. In addition, both seem to be responsible for making critical niche components — HFSCs produce the K6+ inner bulge cells, and ISCs make the Paneth cells. In this regard, mammalian HFSCs and ISCs seem to differ significantly from D. melanogaster CySCs, which might contribute to their own niche only after hub formation.
Despite these similarities, stem cell progeny of the hair follicle and intestine display distinct effects on their self-renewing stem cell parents: K6+ bulge cells enhance HFSC quiescence, whereas Paneth cells promote ISC proliferation. These differences also reflect the stereotypical behaviours of the stem cells in their resident compartments: the HFSCs in the bulge are usually in a state of quiescence, whereas ISCs at the crypt base rapidly cycle. Although additional studies are still necessary, it is tempting to speculate that, by designing key niche components from downstream committed progeny, stem cells may receive a blueprint for the cycling behaviour needed for sustaining homeostasis within the tissue. Given that these epithelial tissues must also respond to injury, the ability of stem cells to receive cues from committed progeny adds flexibility to the blueprint, further enabling the stem cells to strike the requisite balance between proliferation and quiescence within their niche.
It is also worth noting that HFSC or ISC populations with different cycling properties are positioned at distinct locations, which might reinforce their cycling behaviours: in contrast to the bulge HFSCs, the cells of the hair germ do not make direct cell–cell contact with inhibitory K6+ bulge cells, but rather they are adjacent to the dermal papilla activation centre. Similarly, the +4 slow-cycling ISCs reside above the proliferation-promoting Paneth cells and are closer to the BMP-expressing mesenchymal cells at the crypt–villus border. Such features suggest that microenvironment has an impact on stem cell activity.
The haematopoietic system
Thus far, we have introduced several examples in which the progeny of stem cells regulate the cycling frequencies of their parents. The haematopoietic stem cell (HSC) system brings new complexities to the relationship between stem cells and their niches. Most adult HSCs reside in bone marrow23,24, where they become activated to replenish the myeloid and lymphoid lineages of the blood and the immune system94. Unlike most other stem cells, which reside either within their niche or within a relatively limited range of travelling distance surrounding the niche, HSCs are extraordinarily mobile. During homeostasis, HSCs often travel from one bone marrow compartment to another95. Under stress conditions when the bone marrow cannot sustain sufficient haematopoiesis, such as in myelofibrosis, HSCs can even travel to the spleen or liver.
The HSC niche
Accompanying the unusually mobile and dynamic nature of its key residents is a complexity to the HSC niche that has not been seen in other stem cell systems. In addition to well-established niche components, such as osteoblasts and bone marrow vasculature21,96–100, osteoblast progenitors, adipocytes, mesenchymal cells and non-myelinating Schwann cells within the bone marrow have recently been shown to influence HSC behaviour101–105.
It has also been postulated that the location of HSCs directly affects their cycling behaviour, presumably because of the different environmental stimuli they encounter at each location. In this regard, live-imaging studies suggest that HSCs are more quiescent when they are closer to the endosteal lining, which is enriched for osteoblasts, osteoclasts and stromal fibroblasts. By contrast, more active HSCs tend to be closer to the perivascular niche, which is more centrally located within the bone marrow and marked by perivascular mesenchymal stem cells (MSCs), sinusoid endothelial cells and neural cells23,24.
As the number of cell types known to regulate HSCs continues to increase, dissecting the complex crosstalk involved and the differentiation status of other niche lineage cells becomes a major challenge. Osteoblasts were among the first niche residents to be characterized. Paralleling the Paneth cell–ISC connection, when osteoblast numbers are increased, HSCs expand concomitantly96,97. Conversely, depletion of osteoblasts results in a loss of HSCs106,107. By contrast, osteoclasts seem to be dispensable for HSC maintenance108. The most dormant, long-lived HSCs seem to reside next to osteoblast progenitor cells (which are known as preosteoblasts). Further supporting a functional role for these progenitors in regulating HSC dormancy, mutational loss of microRNA processing in preosteoblasts, but not in mature osteoblasts, causes HSC hyperproliferation, aberrant haematopoiesis and myelodysplasia104.
On the perivascular side of HSC niche components are nestin+ MSCs, which influence HSC migration and maintenance (see below)102. CXCL12-abundant reticular cells (CAR cells) also affect HSC numbers103. These cells may partially overlap with nestin+ MSCs on the perivascular side but have also been seen in the endosteum. Finally, non-myelinating Schwann cells appear to maintain HSC quiescence by regulating latent TGFβ activation105. This is in contrast to the function of TGFβ in the hair follicle niche, where active TGFβ seems to activate HFSCs70. Although much is still to be learned about these signalling networks, it is already clear that different cell types within both endosteal and perivascular compartments influence HSC behaviour in multifaceted ways (FIG. 5a).
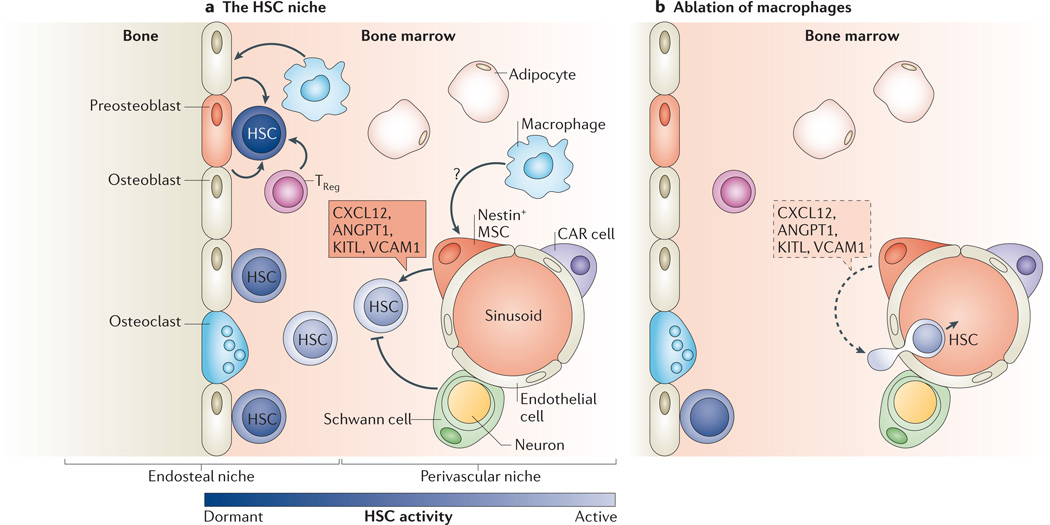
Figure 5. The HSC niche: a rich and complex environment.
a | Most haematopoietic stem cells (HSCs) reside in the bone marrow, which can be subdivided into endosteal and perivascular niches. HSCs located on the endosteal side tend to be more quiescent, whereas HSCs located at the perivascular side are more active. The most dormant HSCs have been reported to locate near osteoblast progenitors (preosteoblasts). With the exception of osteoclasts, all of the cellular components in the diagram have been reported to modulate HSC behaviour. Two of the downstream HSC progeny, regulatory T cells (TReg cells) and macrophages, participate in HSC regulation. TReg cells guard HSCs by making the endosteal niche a possible immune-privileged site, thereby protecting HSCs from immune attack. Macrophages are critical for maintaining HSCs in their bone marrow niche. They appear to do so through an indirect mechanism mediated by nestin+ mesenchymal stem cells (MSCs) and possibly by osteoblasts. b | Ablation of bone marrow macrophages reduces the levels of several key maintenance factors for HSCs, such as CXC chemokine ligand 12 (CXCL12), angiopoietin 1 (ANGPT1), KIT ligand (KITL) and vascular cell adhesion molecule 1 (VCAM1), that are expressed in paracrine by the nestin+ MSCs. Exactly how macrophages signal to the nestin+ MSCs to govern this exchange of signals remains to be identified (and thus is represented by a question mark). CAR cell, CXCL12-abundant reticular cell.
HSC progeny are HSC regulators
Regulatory T cells (TReg cells) are a type of HSC progeny in the lymphoid lineage. They are critical for suppressing immune responses and confining tolerance to self-antigens. TReg cells also mediate immune response suppression at specific immune-privileged sites, where introduction of foreign antigens is tolerated without eliciting an inflammatory immune response.
Immune privilege is thought to be an adaptation that protects vital structures from deleterious damage caused by inflammation. As stem cells are crucial for tissue regeneration, it has been speculated that they might reside within immune-privileged sites. At least for HSCs, this seems to be the case. Thus, transplanted HSCs survive in the bone marrow of non-irradiated syngeneic and allogeneic recipients without eliciting immune response. This effect seems to be attributable to TReg cells within the bone marrow, as their depletion results in fewer surviving donor HSCs. In this regard, TReg cells may act as a shield for HSCs, protecting them from immune attack109.
On the side of myeloid lineage progeny, macrophages also provide interesting feedback to HSCs. It appears that the movement of HSCs is regulated by macrophages located in the bone marrow110,111. Two independent groups used conceptually similar but technically different ablation approaches to study these macrophages. Winkler and colleagues110 targeted ablation of macrophages by two methods: intravenous injection of clodronate-loaded liposomes, which are taken up by macrophages; and a transgenic model that triggered macrophages to undergo FAS-induced apoptosis. Chow and colleagues111 used a CD169–DTR model that expressed DTR in a subset of macrophages, enabling them to induce depletion upon injection with diphtheria toxin. Although different ablation approaches might target different subtypes of macrophages, both groups found that, when macrophages are depleted, large numbers of HSCs mobilize from their bone marrow niche and enter the peripheral bloodstream (FIG. 5b).
How do macrophages influence the localization of HSCs? Although the exact mechanism remains to be elucidated, current evidence suggests that they do so through an indirect fashion. On the perivascular side, this effect seems to involve the nestin+ MSCs in the bone marrow. Purified HSCs home near these nestin+ MSCs when transplanted into hosts and, conversely, depletion of nestin+ MSCs rapidly diminishes HSC numbers102. Upon depletion of macrophages, several genes implicated in HSC retention or maintenance are specifically downregulated within nestin+ MSCs residing within the bone marrow111. These include the genes encoding the secreted factors CXC chemokine ligand 12 (CXCL12; also known as SDF1), angiopoietin 1 (ANGPT1), KIT ligand (KITL; also known as stem cell factor and steel factor) and vascular cell adhesion molecule 1 (VCAM1), and their downregulation suggests that macrophages may affect HSC retention through regulating critical retention factors in nestin+ MSCs. On the endosteal side, ablation of macrophages may affect osteoblast numbers, thereby making the bone marrow cavity a less favourable site for HSCs110,112. The next important step will be to delineate the signals sent from macrophages to nestin+ MSCs and/or osteoblasts to control HSC retention within the bone marrow.
The example of macrophages and HSCs unveils yet another intriguing mechanism for how progeny can influence stem cells. In this case, instead of affecting stem cells directly, the progeny first have an impact on other heterologous niche cell types within the microenvironment and this then affects the behaviour of stem cells. Given the coexistence of multiple heterologous cell types within various stem cell niches, additional examples of these intricate circuits are likely to unfold in the future.
Conclusions and perspectives
In this Review, we present a range of recent examples illustrating how progeny can provide important and diversified feedback mechanisms to regulate their stem cell parents. Together with heterologous niche cell types, they create a unique microenvironment to host the stem cells. Given that the main function of stem cells is to generate tissue, the generation of feedback signals from differentiated progeny to regulate stem cell behaviour becomes a robust strategy to control tissue homeostasis. In the case of the intestine, the equilibrium between ISCs and Paneth cells ensures that a constant number of stem cells are maintained in the face of rapid tissue regeneration. In the case of the hair follicle, the stop signal imparted to stem cells by the K6+ bulge cells at the end of the hair cycle provides an explanation for how the stem cell niche can remain dormant in telogen for weeks on end despite stimulatory cues from the dermal papilla. It also provides an intriguing insight into why hairs grow back again when plucked and why skin tumours, for example squamous cell carcinomas, which lack the inner bulge structure, might unleash a more proliferative state to their malignant stem cell residents.
A final note that is worthy of mention is the advantage of this regulatory circuitry in wound repair. Most tissues of the body must mobilize stem cells quickly in response to injury. But, once the tissue has been repaired, the stem cells must also be able to return to their normal proliferative state. The dependency of stem cells on their downstream progeny provides a natural means for telling stem cells when to expand tissue growth and when to stop during normal homeostasis. Upon wounding, when these regulatory progeny are damaged, the niche environment changes accordingly, allowing stem cells to take immediate action and repair the tissue.
Given the near-universal requirement to precisely control stem cell activity in adult tissues, the examples we present here are likely to be the ‘tip of the iceberg’ in the study of niche dynamics between stem cells and their progeny. Studies regarding stem cell niches are increasingly unravelling new concepts in basic stem cell biology and are beginning to have an impact on regenerative medicine. And stem cell progeny have surfaced as indispensable components that researchers must take into account when trying to tease out the complexity of the niche. With increasing evidence that tumour-initiating, or so-called cancer stem cells, also partake in extensive dialogue with their surroundings, it seems that it is just a matter of time before incidences of crosstalk between cancer stem cells and their local downstream progeny emerge. By better understanding the niche composition and mapping the signals within the microenvironment with higher precision, it may be possible in the future to harness this information and design effective strategies to promote stem cell proliferation and regeneration following injury or degeneration, and to restrict it in malignant progression.
Note added in proof
While this review was in press, a study was published reporting a feedback regulation by progeny in the D. melanogaster haematopoietic niche. Signals from differentiating haemocytes were found to regulate haematopoietic progenitor quiescence117. This provides yet another example of this emerging regulatory mechanism governing stem cell behaviours in their niches.
Acknowledgements
We thank B. Keyes, T. Chen and M. Genander for critical readings and comments of the manuscript. Y.-C.H. was a Starr Stem Cell Scholars postdoctoral fellow and is now supported by a New York Stem Cell Foundation–Druckenmiller fellowship. E.F. is a Howard Hughes Medical Institute Investigator. This work is supported by a grant from the US National Institutes of Health (R01-AR050452).
Glossary
- Basement membrane
A sheet-like structure that is composed of extracellular matrix and separates the cavity and surfaces of an organ.
- Cancer stem cells
Long-term self-renewing cells within a tumour that are responsible for initiating the cancer and propagating it. The term does not reflect the origin of these cells or their molecular similarities to normal stem cells. Rather, these cells are tumour-initiating cells and can execute a differentiation programme, but it is an aberrant one.
- Bulge
A protruding structure of the hair follicle in which hair follicle and melanocyte stem cells reside.
- Integrins
A family of cell adhesion receptors that mediate either cell–cell interactions or cell–extracellular matrix interactions. Integrins are heterodimers with two distinct subunits, which are known as the α-subunit and the β-subunit.
- Hair shaft
A terminally differentiated structure that protrudes out from the skin surface as a hair.
- Transient amplifying progeny
A special population of stem cell progeny responsible for the bulk of tissue growth. Transient amplifying cells are larger in quantity and are capable of massive expansion and proliferation within in a short time. However, they can only undergo a finite number of divisions.
- Crypt
A moat-like tubular invagination of the intestinal epithelium. Crypts contain intestinal stem cells and Paneth cells.
- Villi
Finger-like structures that project into the lumen of the intestine. Villi contain the absorptive enterocytes and mucus-secreting goblet cells. These cells live for a few days before they die and are shed from the intestinal epithelium.
- Myeloid
A lineage containing macrophages, monocytes, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes and dendritic cells.
- Lymphoid
A lineage containing all of the T cells, B cells and natural killer cells.
- Myelofibrosis
A disease in which fibrous scars accumulate in the bone marrow cavity.
- Non-myelinating Schwann cells
Schwann cells are the glia cells of the peripheral nerve system. Non-myelinating Schwann cells lack a myelin sheath. They are often found to wrap around axons with a smaller diameter and are important for the survival and function of neurons
- Endosteal lining
A thin layer of connective tissue that lines the medullary cavity of a bone.
- Mesenchymal stem cells
(MSCs). Multipotent stem cells capable of giving rise to a wide range of mesenchymal cells, including adipocytes, chondrocytes and osteoblasts.
- Myelodysplasia
A group of disorders in which the bone marrow does not function normally and insufficient numbers of blood cells are produced.
- Syngeneic
When donors are genetically identical or at least immunologically compatible with recipients.
- Allogeneic
When donors are from the same species but are genetically different from recipients.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Elaine Fuchs’s homepage: http://lab.rockefeller.edu/fuchs
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 2. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. Marks slow-cycling cells located in the bulge region and suggests that they might be HFSCs.
- 3. Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. Describes the generation of a mouse model combining two transgenes to fluorescently tag slow-cycling cells, which has been widely applied to other systems, including the HSCs as in references 26 and 80. Also reports the transcriptional profiling that identified genes preferentially expressed by HFSCs.
- 4.Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- 5.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 8.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 9.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li LH, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz OH, et al. Pten-dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 14.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 15.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl Acad. Sci. USA. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. Postulates the existence of a stem cell niche.
- 17.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 18.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 19. Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. References 17–19 demonstrate the existence of niches for D. melanogaster germline stem cells and describe the signalling events involved. References 17 identifies ‘cap cells’ as key GSC niche components in the ovary. References 18 and 19 define the hub as the niche for testis GSCs.
- 20.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Chan CKF, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie YC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. References 23 and 24 apply live-imaging techniques to visualize mouse HSCs in their native bone marrow niches. References 24 images murine bones ex vivo, whereas reference 23 takes an in vivo approach to image cells within the bone marrow.
- 25. Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. Shows, using Cre–ER–Rosa–LacZ lineage tracing that LGR5+ cells at the crypt base are fast-cycling ISCs.
- 26.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 28.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 29.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 30.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flaherty MS, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issigonis M, et al. JAK–STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–156. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leatherman JL, DiNardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nature Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 35.Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr. Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- 37. Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–1696. doi: 10.1242/dev.057364. References 35 and 37 demonstrate that, under some circumstances, progeny of CySCs can differentiate into hub cells. Reference 35 observes this contribution during steady state. Reference 37 observes this conversion mainly when the CySCs carry mutations in the lines gene.
- 38.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nature Biotech. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 39. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. Similarly to reference 3, references 38 and 39 delineate transcriptional profiling that identified genes preferentially expressed by HFSCs.
- 40.Vidal VP, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 41.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen H, et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 45.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. Details combinatorial pulse-chase and lineage-tracing experiments to monitor HFSCs after exiting the bulge. Reveals that some committed HFSC progeny return to the niche and confer inhibitory signals that help maintain HFSC quiescence in the bulge.
- 47.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 49.Tanimura S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 50.Rabbani P, et al. Coordinated activation of wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujiwara H, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived Sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lien WH, et al. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–232. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Botchkarev VA, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nature Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 57.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller-Rover S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 62.Lo Celso C, Prowse DM, Watt FM. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- 63.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl Acad. Sci. USA. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang JW, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 67.Andl T, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 68.Plikus MV, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 70.Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 72.Inoue-Narita T, et al. Pten deficiency in melanocytes results in resistance to hair graying and susceptibility to carcinogen-induced melanomagenesis. Cancer Res. 2008;68:5760–5768. doi: 10.1158/0008-5472.CAN-08-0889. [DOI] [PubMed] [Google Scholar]
- 73.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 74.He XC, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nature Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 75.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell. Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 76.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montgomery RK, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl Acad. Sci. USA. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takeda N, et al. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. Applying similar strategies to reference 25, references 76–78 show that slow-cycling cells located at the +4 position are also ISCs. Reference 78 shows that the two ISC populations are interchangeable.
- 79.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foudi A, et al. Analysis of histone 2B–GFP retention reveals slowly cycling hematopoietic stem cells. Nature Biotech. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gregorieff A, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. Shows that, when Paneth cell numbers are reduced by either mutations or ablations, LGR5+ ISC numbers are reduced concomitantly, suggesting an interdependency of ISCs on one of their downstream lineages.
- 84.Mustata RC, et al. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 2011;12:558–564. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shroyer NF, Wallis D, Venken KJT, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math 1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bastide P, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mori-Akiyama Y, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 88.Garabedian EM, Roberts LJJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 89.van der Flier LG, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 90.Sato T, et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 91.de Lau W, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 92.Glinka A, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haramis AP, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 94.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 95.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 96.Zhang JW, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 97.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 98.Hattori K, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nature Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 100.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omatsu Y, et al. The essential functions of adipoosteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Raaijmakers MH, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamazaki S, et al. Nonmyelinating schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 106.Visnjic D, et al. Conditional ablation of the osteoblast lineage in Col2.3Δtk transgenic mice. J. Bone Miner. Res. 2001;16:2222–2231. doi: 10.1359/jbmr.2001.16.12.2222. [DOI] [PubMed] [Google Scholar]
- 107.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 108.Miyamoto K, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J. Exp. Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. Provides evidence suggesting that TReg cells protect their HSC parents from immune attack by making the endosteal niche an immune-privileged site.
- 110.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 111. Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011;208:261–271. doi: 10.1084/jem.20101688. References 110 and 111 show that macrophages, which are a type of HSC progeny of the myeloid lineage, function in retaining HSCs in their bone marrow niche.
- 112.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J. Exp. Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wieschaus E, Szabad J. The development and function of the female germ line in Drosophila melanogaster : a cell lineage study. Dev. Biol. 1979;68:29–46. doi: 10.1016/0012-1606(79)90241-0. [DOI] [PubMed] [Google Scholar]
- 114.Lin H, Spradling AC. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 1993;159:140–152. doi: 10.1006/dbio.1993.1228. [DOI] [PubMed] [Google Scholar]
- 115.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 116.Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- 117.Mondal BC, et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]