Abstract
Objective
We examined gene expression profiles in peripheral blood leukocytes (PBL) of patients with osteoarthritis (OA) in comparison with non-OA controls to evaluate whether gene expression profiles could serve as biomarkers of symptomatic knee OA. We also determined whether candidate genomic biomarkers (PBL expression of inflammatory genes) predict increased risk of disease progression in subjects with symptomatic radiographic knee OA
Methods
Three independent cohorts of patients with knee OA and non-OA controls were studied: two cohorts (“learning cohort” and “validation cohort”) recruited at New York University Hospital for Joint Diseases (NYUHJD) and one (“validation cohort”) at Duke University Medical Center. PBL gene expression was assessed using Affymetrix microarray and confirmed by QPCR. Radiographic progression at 2 years was assessed in 86 patients
Results
We identified 173 genes significantly up- or down-regulated (≥1.5-fold change) in OA PBL, at a False Discovery Rate (FDR) of 5%. Cluster analysis revealed two distinct subclasses among these OA patients: those with increased expression (≥2-fold) of IL-1β compared to controls, and those with expression comparable to controls. Overexpression of IL-1β in OA subclasses was validated using QPCR (p<0.0001) in all three cohorts. Patients with the inflammatory “IL-1β signature” had higher pain scores, decreased function and were at higher risk for radiographic progression.
Conclusion
PBLs from patients with symptomatic knee OA display a characteristic transcriptome profile. Moreover, increased expression of IL-1β identifies a subset of OA patients with increased pain who are at higher risk for radiographic progression.
Keywords: osteoarthritis, interleukin-1, leukocyte gene expression, progression, pain
Introduction
Osteoarthritis (OA) is the most common adult joint disease, increasing in frequency and severity in all aging populations, with an estimated U.S. prevalence of over 25 million affected adults (1). Disease progression is associated with cartilage degradation, joint space narrowing (JSN), and loss of function. While radiographic progression has highlighted JSN, the emergence of magnetic resonance imaging (MRI) has underscored the involvement of multiple joint tissues in OA, particularly subchondral bone, menisci, and synovium (2-4). Although traditionally considered a non-inflammatory joint disease, it is now well-appreciated that inflammatory mediators are produced by articular tissues in OA and have been implicated in disease pathogenesis (5;6). Indeed, a growing body of evidence supports a role of IL-1, which promotes cartilage degradation (6), resorption of bone (7), and disease progression in animal models of OA (8;9). Furthermore, low innate capacity to produce the pro-inflammatory cytokines IL-1beta and IL-6 is associated with the absence of OA in old age (10). Additionally, we have recently reported that genetic polymorphisms in IL1 receptor antagonists (IL-1Ra) associate with disease severity in knee OA (11;12).
There is currently great interest in the field of OA to identify biomarkers that provide a method for earlier diagnosis and identify patients at higher risk for disease progression. A useful classification system, called BIPEDs, categorizes the major types of OA biomarkers into 5 categories: Burden of disease, Investigational, Prognostic, Efficacy of Intervention, Diagnostic and Safety biomarkers (13). In the studies here reported, we assessed the gene expression of circulating peripheral blood leukocytes (PBLs) as potential biomarkers in OA. We hypothesized that PBLs, which traffic through the tissues of the inflamed joint, are activated by exposure to IL-1β and other locally-produced inflammatory mediators. To assess this hypothesis, we examined PBL gene expression profiles using microarrays and QPCR in controls and three independent populations of patients with knee OA.
Our data indicate that differential PBL gene expression profiles may serve as biomarkers for both diagnosis and, perhaps more importantly, identification of subclasses of knee OA patients at higher risk for disease progression.
Patients and Methods
Patients
Three independent cohorts of patients were examined: two from NYUHJD (designated “Learning Cohort” and “NYUHJD Validation Cohort”) and the “The Duke Validation Cohort.” Studies were approved by the Institutional Review Boards (IRB) of the NYU School of Medicine and Duke University, and all participants provided written informed consent prior to initiation of the studies.
NYUHJD Learning Cohort
Patients were diagnosed with knee OA by the referring physicians, according to the 1986 American College of Rheumatology (ACR) classification, and met clinical plus either radiographic or laboratory criteria for the diagnosis of idiopathic OA of the knee. Demographics, comorbidities, medications, number and location of joints affected (by both patient and physician report) were collected. We excluded patients with any other form of arthritis; body mass index (BMI) >33; any disorder requiring the use of systemic corticosteroids; history of bilateral knee replacements; major co-morbidities including diabetes mellitus, non-cutaneous cancer within 5 years of screening, chronic hepatic or renal disease, chronic infectious disease, congestive heart failure; and hyaluronan and/or corticosteroid injection to the affected knee within 3 months of screening. Non-OA controls had no clinical signs or symptoms of any arthritis. We assessed pain, functional status and quality of life with the Western Ontario and McMaster Osteoarthritis Index (WOMAC) (14), Short Form Health Survey (SF-36) (15), and Multidimensional Health Assessment Questionnaire (MDHAQ) (16); within the MDHAQ are modules for the patient global assessment of their disease and for visual analog scale (VAS) pain assessment.
The NYUHJD Validation Cohort
As part of an NIH-funded study, a separate cohort of 86 patients were followed for 24 months who met ACR clinical symptomatic criteria (17) and radiographic criteria [Kellgren-Lawrence (KL) grade ≥1] (18) for symptomatic knee OA. Exclusion criteria were as noted above. All patients underwent bilateral standardized weight bearing fixed-flexion posteroanterior (PA) knee radiographs using the SynaFlexer™ X-ray positioning frame (Synarc). We also screened 41 age-matched healthy controls, and among these recruited 20 subjects who had KL radiographic score <1 and no knee pain, of whom 12 were available for the analyses reported here. All patients were examined by an NYUHJD investigator every 6 months in this 24-month study. Radiographic assessments at baseline and 24 months include bilateral (signal and non-signal knee) KL determination, quantitative measurement of medial and lateral joint space width (JSW), medial and lateral JSN, osteophytes, medial tibial/lateral femoral subchondral sclerosis, and medial tibial attrition using the OARSI atlas, by two musculoskeletal radiologists blinded to patient information. Kappas for inter-rater agreement were 0.85 and 0.77 for KL scores of the right and left knees, respectively, and >0.85 for most other radiographic outcome measures. Concordance correlation coefficients were >0.90 for JSW measurements. JSW was measured at the narrowest portion of the joint space via electronic calipers and a medical monitor; the average value between the two readers was used in the statistical analysis. We collected genomic DNA and RNA from peripheral blood mononuclear cells (PBMCs) from each OA patient and non-OA control.
Duke Validation Cohort
A third independent population was selected from among 159 participants (118 female, 41 male) enrolled in the NIH-sponsored Strategies to Predict Osteoarthritis Progression (POP) study (N=56) (19). Excluding patients with BMI ≥33, this study provided data on 36 patients. The POP study patients were recruited from the Durham, NC area under a protocol approved by the Duke University IRB and in accordance with the policies of the Duke University Medical Center. Participants were recruited who met the ACR criteria for symptomatic OA of at least one knee (18). Exclusion criteria were similar to above, as reported (19). For gene expression studies by TaqMan QPCR analysis from the Duke patients, PAXgene samples were utilized.
Sample collection
Blood samples in the NYUHJD cohorts were collected in pyrogen-free heparinized tubes for isolation of PBMCs and plasma, as well as serum collection tubes. PBMCs were isolated as described (20). PAXgene tubes were collected for RNA in all three cohorts. Blood was processed within 30 minutes of collection.
Total RNA isolation and gene expression analysis
Total RNA from PBMCs was isolated using Qiagen RNeasy column. Total RNA from PAXgene tubes was isolated using a PreAnalytiX blood RNA isolation kit (Qiagen Biosciences). Isolated total RNA was stored in aliquots at −70°C. It should be noted that in contrast to the PBMCs isolated from the NYUHJD patients by Ficoll-Hypaque density centrifugation, which were mononuclear leukocytes, neutrophils are the predominant PBL cell type that contribute to mRNA isolated from PAXgene tubes.
Real Time PCR (QPCR)
Total RNA (1 μg) was primed using oligo (dT) 18 primers and cDNA synthesized using the Clontech cDNA synthesis kit (Clontech). Predesigned TaqMan primer sets were purchased from Applied Biosystems. Real-time PCR reactions were performed as described (21).
Labeling and hybridization of microarray
Five micrograms of total RNA were used for the first strand synthesis using a T7-(dT)24 oligomer. Complementary RNA was synthesized using ENZO kit (Affymetrix) and purified using the Qiagen RNeasy kit, fragmented at 95°C for 35 min for target preparation, and hybridized against Human U133A microarray (Affymetrix).
Normalization of microarray data
Raw expression data from array scans were pre-processed using the Affy package from Bioconductor (22), normalized using the RMA method of Irizarry et al (23), and analyzed further using the R language for statistical computing (24). Genes with less than 70% reliable expression measurement (“present” call in Affymetrix) were filtered out, leaving 8700 genes for analysis.
Statistical methods
Significance Analysis of Microarrays (SAM) implemented in the R package samr was used to find genes differentially expressed between non-OA controls and OA subjects (25). SAM uses the False Discovery Rate (FDR) approach to control for multiple comparisons (26).
In order to identify and validate OA subclasses, the training/test approach was used. In the training set, complete-linkage hierarchical clustering was used to identify OA subclasses based on the expression of a pre-selected set of cytokine genes. Cluster and TreeView packages were used for cluster analysis (27). Nearest centroid classification was used to classify test set samples into subclasses of OA. Briefly, nearest centroid classification method computes a centroid for each OA subclass in the training set, based on the expression of the pre-selected set of cytokine genes, and assigns each test set sample to the OA subclass whose centroid it is closest to in squared distance.
The “nearest shrunken centroid” method implemented in the R package pamr (Prediction Analysis of Microarrays) was used to construct a gene expression classifier of OA and that of OA subclasses (28). The nearest shrunken centroid classification makes one important modification to standard nearest centroid classification. It shrinks each of the class centroids toward the overall centroid for all classes by an amount called the threshold. This shrinkage consists of moving the centroid towards zero by threshold, setting it equal to zero if it hits zero. Shrinkage makes the classifier more accurate by reducing the effect of noisy genes and performs automatic gene selection. The value of the threshold is determined by cross-validation in pamr. The predictive accuracy of the gene expression classifier of OA subclasses was estimated using the test set.
The method of pre-validation (PV) was used to assess the significance of the microarray biomarker of OA while controlling for age, BMI and other demographic characteristics in a logistic regression model (29). PV outputs a prediction for each patient based on the model that is estimated without using that patient's data, thus reducing bias associated with reuse of the data.
Box-Cox transformations were used to normalize variables prior to using statistical tests and regression models. All statistical analyses were performed using the R language for statistical computing (24) and Bioconductor packages (22). Linear (logistic) regression methods were used to compare the groups (OAIL-1, OAnl). Spearman's correlation coefficient r was used for different correlation analysis.
Results
Study cohorts
Three independent cohorts of patients were examined: two from NYUHJD (“Learning Cohort” and “NYUHJD Validation Cohort”) and the “Duke Validation Cohort.” The strategy for identifying differentially expressed PBMC genes in the NYUHJD Learning Cohort, and validating expression of those genes in the NYUHJD Validation and Duke Validation Cohorts, is outlined in Figure 1.
Figure 1.
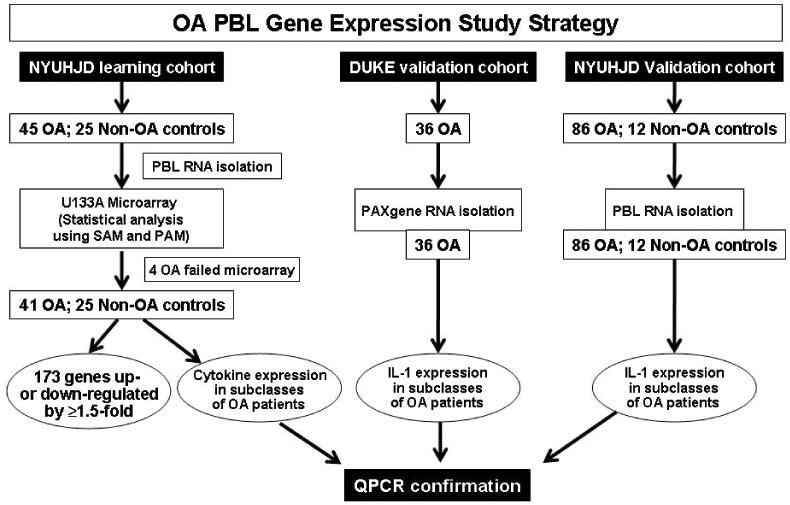
Osteoarthritis peripheral blood leukocyte (OA PBMC) gene expression study strategy. Three cohorts were studied. The original study cohort (NYUHJD Learning Cohort) recruited 45 OA patients and 25 non-OA controls. Five microgram of total RNA isolated from PBMC was used for biotin cRNA synthesis and hybridized against Affymetrix human U133A chip; microarray assessment was accomplished for 41 OA patients and 25 non-OA controls. Using Significance Analysis of Microarrays (SAM), we identified 173 genes significantly up- or down-regulated (≥1.5-fold change) in the NYUHJD Learning Cohort OA PBMC, at a False Discovery Rate (FDR) of 5%. Two additional cohorts, NYUHJD Validation Cohort and Duke Validation Cohort, were used for validation of IL-1β expression by QPCR.
NYUHJD Learning Cohort
We recruited 45 OA patients and 25 non-OA healthy controls into the NYUHJD Learning Cohort: OA patients were significantly older (p <0.001) and had higher BMI than non-OA healthy controls (p=0.017). Approximately 25% of the subjects were males and 80% were non-Hispanic among OA patients as well as non-OA healthy controls. To address differences in age and BMI we selected a sub-cohort (designated the Matched Sub-cohort) of 19 OA patients and 17 healthy controls, frequency-matched on age, gender, ethnicity and BMI, for further analysis.
PBMC gene expression profile as a diagnostic combinatorial biomarker: Pre-validated Microarray Predictor
Using the nearest shrunken centroid classification (28), we constructed a microarray combinatorial biomarker diagnostic of OA based on the NYUHJD Learning Cohort microarray data. We performed this analysis based on the entire NYUHJD Learning Cohort and repeated it based on the matched sub-cohort. We fitted a logistic regression model of OA with the microarray biomarker alone and in combination with BMI, age and ethnicity as independent variables. To reduce bias associated with re-use of the data, we used the method of pre-validation when assessing the significance of the microarray predictor (29). The pre-validated microarray predictor was significant when alone (p=0.0002) and while controlling for the subject characteristics (p=0.0017). Significant predictors included the differential gene expression profile microarray [odds ratio (OR) 8.51, 95% confidence interval (CI) 2.03-35.7], age (OR 1.10, 95% CI 1.02-1.19) and BMI (OR 1.23, 95% CI 1.02-1.49).
Significant genes
In the NYUHJD Learning Cohort, we identified 332 upregulated genes and 193 downregulated genes in OA using a FDR of 5% and with at least a 1.25-fold change. Using the matched sub-cohort, we identified 828 upregulated genes and 448 downregulated genes in OA by the same criteria. Seventy-three percent of the upregulated genes and 62% of down-regulated genes identified using data on all subjects were also found to be significant based on the matched sub-cohort data. Among the most significant genes were junB, CDC42, Granulin, MMP-9 and cyclin D2. Using the method of cross-validation, we estimated that this gene expression pattern has sensitivity of 89% and specificity of 76% for an OA diagnosis [see Supplemental Tables 1 and 2 (ST1 and ST2)].
PBMC inflammatory gene expression identifies OA subclasses
Given the role that inflammation in joint tissues has been shown to play, we determined whether PBMCs were “activated,” reflecting exposure to inflammatory stimuli as these cells traversed diseased synovium and bone. Therefore, to identify subclasses of OA, we randomly divided the NYUHJD Learning Cohort OA samples into a separate training set (n=21) and test set (n=20). The samples had been pre-stratified to ensure that the two sets were frequency matched on sex, ethnicity and BMI. In the training set, we used complete-linkage hierarchical clustering to identify subclasses of OA based on the expression of 21 pre-selected cytokine genes (see Table 1). Two subclasses of OA were identified in the training set: cytokine overexpressors (OAIL-1) and cytokine underexpressors (OAnl). OAIL-1 class had elevated levels of IL-1β (up 6.56-fold), IL-8 (up 2-fold), COX-2 (up 2.75-fold), and chemokines GRO2 (up 5.37-fold), macrophage inflammatory protein-1α (MIP-1α) (up 6-fold) and MIP-1β (up 2.75-fold). Based on the gene expression of the 21 cytokines, we computed the centroids C1 and C2 of OA classes in the training set and labeled each OA test sample as OAIL-1 or OAnl according to whether it was most correlated with C1 or C2 (nearest centroid classification). Therefore, we defined OA classes using cluster analysis based solely on the expression of the 21 cytokine genes. Presented in Figure 2A is the hierarchical clustering diagram of all OA samples based on the expression of the 21 pre-selected cytokine genes. The centroids of the two classes of OA and on-OA controls are depicted in Figure 2B.
Table 1.
Cytokine genes used to define osteoarthritis (OA) subclasses.
| Gene ID | Gene name |
|---|---|
| 39402_at | interleukin 1, beta |
| 211506_s_at | interleukin 8 |
| 204748_at | prostaglandin-endoperoxide synthase 2 (COX-2) |
| 202859_x_at | interleukin 8 |
| 201044_x_at | dual specificity phosphatase 1 |
| 212657_s_at | interleukin 1 receptor antagonist |
| 212659_s_at | interleukin 1 receptor antagonist |
| 209774_x_at | chemokine (C-X-C motif) ligand 2 |
| 205114_s_at | chemokine (C-C motif) ligand 3-like, centromeric |
| 204103_at | CCL4 (MIP-1 beta) |
| 202687_s_at | tumor necrosis factor (ligand) superfamily, member 10 |
| 202688_at | tumor necrosis factor (ligand) superfamily, member 10 |
| 203508_at | tumor necrosis factor receptor superfamily, member 1B |
| 204780_s_at | tumor necrosis factor receptor superfamily, member 6 |
| 204781_s_at | tumor necrosis factor receptor superfamily, member 6 |
| 207643_s_at | tumor necrosis factor receptor superfamily, member 1A |
| 207892_at | tumor necrosis factor (ligand) superfamily, member 5 |
| 209295_at | tumor necrosis factor receptor superfamily, member 10b |
| 209499_x_at | tumor necrosis factor (ligand) superfamily, member 12-member 13 |
| 209500_x_at | tumor necrosis factor (ligand) superfamily, member 12-member 13 |
| 201380_at | cartilage associated protein |
Figure 2.
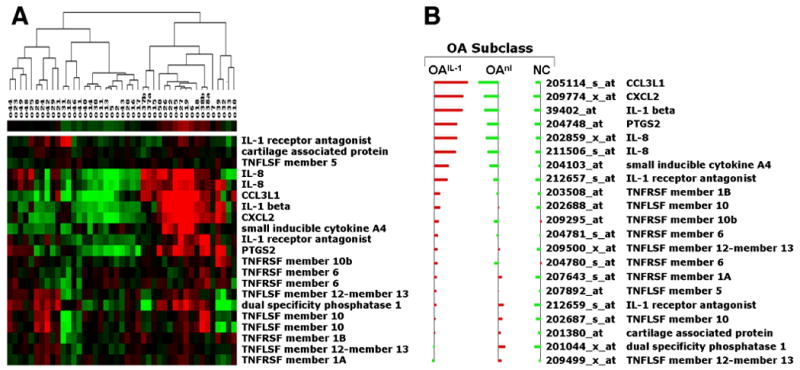
Hierarchical clustering of OA samples based on gene expression of 21 selected inflammatory genes. A, Hierarchical clustering identified two subclasses of OA based on gene expression of 21 selected cytokines: cytokine overexpressors (OAIL-1) and cytokine underexpressors (OAnl). The cytokines are IL-1β, IL-8, COX-2 and chemokines GRO2, cytokine A3 (MIP-1α) and cytokine A4 (MIP-1β). B, Centroids of OA subclasses. The horizontal bars represent the average gene expression for each of the three classes: OAIL-1, OAnl and non-OA controls (NC). The red bars indicate positive average expression while the green bars indicate negative average expression. Abbreviations: CCL3L1, chemokine (C-C) motif ligand 3-like protein 1; CXCL2, chemokine C-X-C motif ligand 2; IL, interleukin; PTGS2, prostaglandin-endoperoxide synthase 2; TNF, tumor necrosis factor; TNFLSF, TNF ligand superfamily; TNFRSF, TNF receptor superfamily.
Cross-validated gene expression (excluding 21 cytokine genes) predictor of OA classes
Using the method of nearest shrunken centroids, we constructed a 10-fold cross-validated predictor of these cluster-defined subclasses of OA based on the training set and excluding the 21 cluster-defining inflammatory genes, in order to find whether other genes or probe sets can stratify subsets of OA patients. The resulting microarray predictor of OA subclasses consisted of eight genes, including cytokine genes such as IL-1β (a different probe set) and TNF inducible proteins [see Supplemental Table 3 (ST3)]. We evaluated the performance of the resulting 8-gene predictor of OA subclass using the test set. The training, cross-validated and test set error of this gene expression predictor of OA subclass was estimated to be over 96%.
Analysis of differentially expressed cytokines by real time RT-PCR
TaqMan real-time QPCR was performed to confirm the findings based on microarrays. The pre-designed primers were purchased from Applied Biosystems. In this study, we analyzed expression of three different house-keeping genes, actin (act), glycerolaldehyde-3 phosphate dehydrogenase (GAPDH) and ribosomal protein large PO (RPLPO). Among the three, only GAPDH showed no significant difference between the non-OA control and OA group (data not shown). The “inflammatory” genes IL-1β, TNFα, IL-6, IL-8, COX-1 and COX-2 were analyzed. Relative expression levels of each gene were calculated using the delta-delta comparative CT method (21). As observed by microarray data (Figure 3), significantly elevated levels of IL-1β, TNFα, IL-6, IL-8 and COX-2 expression were observed in the OAIL-1 as compared to the OAnl subclass (Figure 3A). Relative expression levels of inflammatory genes quantitated by QPCR in OA PBMCs (NYUHJD Learning Cohort) were correlated with pain assessment scores in the linear regression model while controlling for age, BMI, gender and ethnicity. Among the inflammatory genes, only IL-1β and IL-6 levels were significantly associated with pain (r=0.38, p=0.02 and r=0.44, p=0.04, respectively).
Figure 3.
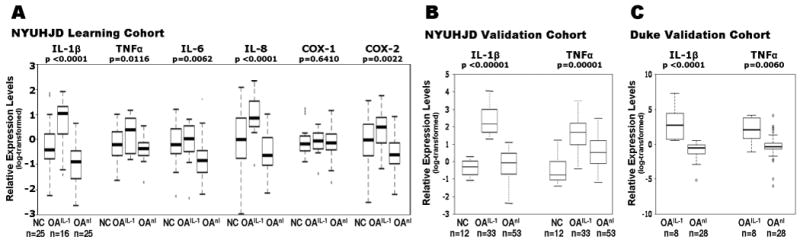
Upregulation of inflammatory genes in the NYUHJD Learning Cohort and NYUHJD and Duke Validation Cohorts. A, Real Time PCR analysis: relative expression levels of inflammatory gene (IL-1β, TNFα, IL-8, IL-6, COX-1 and COX-2) expression in OA PBMCs (NYUHJD Learning Cohort). The raw QPCR data were normalized against housekeeping gene GAPDH, and relative expression levels were calculated using the delta-delta CT method (22). These data show that five of these genes are differentially over-expressed in the OAIL-1 group as compared to non-OA controls (NC) and OAnl. B and C, distributions of IL-1β and TNFα expression levels by group in two validation cohorts. Real Time PCR analysis: relative expression levels of IL-1β in (B) the NYUHJD Validation Ccohort and (C) Duke Validation Cohort by group. These data show that the IL-1β gene is differentially overexpressed in the OAIL-1group as compared to non-OA controls (NC) and OAnl. Box-Cox transformations were applied to normalize variables prior to regression modeling.
Elevated inflammatory gene expression in OA PBMCs confirmed in two independent cohorts
To further confirm the elevated levels of both genes as diagnostic biomarkers for a specific OA subset, we used two different additional independent cohorts of OA patients 1) The Duke Validation Cohort consisted of 36 OA patients and no controls; the Duke OA patients had higher mean BMI than the patients in the other cohorts (Table 2). 2) The NYUHJD Validation Cohort consisted of 86 OA patients and 12 non-OA controls (Table 2). As noted, we defined OAIL-1 and OAnl subclasses in the validation cohorts based on the samples IL-1 expression using the 90% quantile method.
Table 2.
Comparison of osteoarthritis (OA) subclasses with respect to various demographic and clinical characteristics.
| NYUHJD Learning Cohort | Duke Validation Cohort | NYUHJD Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| OAIL-1 (n = 16) |
OAnl (n = 25) |
NC (n = 25) |
OAIL-1 (n = 8) |
OAnl (n = 28) |
OA IL-1 (n=33) |
OAnl (n=53) |
NC (n=12) |
|
| Gender: n (%) | ||||||||
| Male | 2 (12.5%) |
8 (32.0%) |
7 (28.0%) |
1 (12.5%) |
7 (25.0%) |
11 (33.3%) |
24 (45.3%) |
8 (66.7%) |
| Female | 14 (87.5%) |
17 (68.0%) |
18 (72.0%) |
7 (87.5%) |
21 (75.0%) |
22 (66.7%) |
29 (54.7%) |
4 (33.3%) |
| Ethnicity: n (%) | ||||||||
| Hispanic | 3 (18.8%) |
7 (28.0%) |
5 (20.0%) |
1 (12.5%) |
0 | 6 (18.2%) |
6 (11.3%) |
0 |
| Non-Hispanic | 13 (81.3%) |
18 (72.0%) |
20 (80.0%) |
7 (87.5%) |
28 (100%) |
27 (81.8%) |
47 (88.7%) |
12 (100%) |
|
Age: mean (SD) |
64.0 (11.4) |
67.6 (10.0) |
54.1 (9.4) |
55.4 (13.7) |
64.4 (12.3) |
59.2 (9.0) |
63.2 (9.2) |
59.7 (9.2) |
|
BMI: mean (SD) |
25.41 (3.2) |
27.71 (3.3) |
24.5 (3.5) |
29.7 (2.25) |
27.6 (3.15) |
27.1 (3.45) |
26.5 (3.5) |
25.4 (3.7) |
Difference statistically significant (p = 0.04, p = 0.0035 in females) using two-sided rank-sum test.
BMI, body mass index; NC, normal controls; OAIL-1, osteoarthritis subclass with increased IL-1β expression; OAnl, osteoarthritis subclass with normal IL-1β expression; SD, standard deviation.
Percentage totals within categories may exceed 100% due to rounding.
As observed in NYUHJD Learning Cohort, elevated expression of IL-1β and TNFα was confirmed in both the NYUHJD and Duke Validation Cohorts (Figure 3B-C). For the NYUHJD Validation Cohort, OA subjects were defined as cytokine overexpressors (OAIL-1 class) if they had elevated expression (at least 2-fold) of IL-1β. Some characteristics of the OAIL-1 and OAnl subclasses of the NYUHJD Learning Cohort were also confirmed in the NYUHJD Validation Cohort. Specifically, pain assessment scores were significantly higher in the OAIL-1 vs OAnl subclass by all three measures (WOMAC pain, 52.16 ± 23.67 vs. 37.05 ± 19.81, p= 0.0034; WOMAC total, 161.78 ± 64.01 vs. 112.18 ± 55.84, p=0.0004; VAS pain, 61.80 ± 25.80 vs. 42.77 ± 28.17, p=0.0069) in the NYUHJD Validation Cohort. Additionally, IL-1 levels were significantly associated with pain assessment scores by all three measures in the linear regression model while controlling for age, BMI, gender and ethnicity (adjusted p=0.0009, 0.01 and 0.02 for pain VAS, WOMAC total and WOMAC pain, respectively) in the NYUHJD Validation Cohort.
The OAIL-1 subset is at increased risk for radiographic progression
Eighty-six patients in the NYUHJD Validation Cohort completed a 24-month study of radiographic progression. Comparing standardized fixed-flexion radiographs read independently by two radiologists (LR, JB), we determined change in JSW (both in millimeters and as >30% change from baseline) and KL score between visit 0 and 24 months. For KL scores, a change in one grade was considered to represent progression. Baseline IL-1 mRNA expression was used to segregate OA patients into two groups, OAIL-1 and OAnl, compared for progression based on JSW at baseline and >30% JSN at 24 months. OAIL-1 patients exhibited greater JSN at 24 months than the OAnl group (0.76 vs 0.15 mm, p <0.02) (Table 3). Since the mean JSW at baseline differed between OAIL-1 (3.8±1.7 mm) and OAnl (2.1±2.1 mm) groups, we separately analyzed those OA patients who had ≥3 mm baseline JSW. In this sub-analysis (Table 3), JSN at baseline remained higher in the OAIL-1 group compared to OAnl group, and OAIL-1 patients exhibited greater JSN at 24 months than the OAnl group (0.89 vs 0.38 mm, p=0.0507). Logistic regression analysis adjusted for BMI, age and gender also showed increased progression in the OAIL-1 group, as assessed by decrease in JSW (i.e., JSN) >30%, observed in 19 of 86 patients [OR=3.2 (1.16-8.66), p <0.03). Thus, using percent change in JSW (JSN >30%), we could identify a top quartile of “fast progressors” who were identified at baseline by increased PBMC expression of IL-1β. Additionally, using the same logistic regression model, the change of KL score in the OAIL-1 group was significantly greater (p<0.004) compared to the OAnl group over 24 months. After controlling for BMI, age and gender, OAIL-1 also exhibited clinical features that differed from OAnl patients, with higher WOMAC pain, decreased physical function and higher VAS pain scores (p=0.0069).
Table 3.
Medial joint space width (JSW) and joint space narrowing (JSN) scores in signal knee at baseline and 24 months follow-up in knee OA patients with increased (OAIL-1) versus normal (OAnl) IL-1β expression in entire NYUHJD Validation Cohort and in patients with baseline JSW ≥3 mm.
| Mean (standard deviation) | p-value1 | ||
|---|---|---|---|
| Entire NYUHJD Validation Cohort | OAIL-1 (n=33) | OAnl (n=53) | |
| JSW (mm) at baseline | 3.87 (1.71) | 2.14 (2.15) | 0.0005 |
| JSW (mm) at 24 months | 3.10 (1.79) | 2.01(2.10) | 0.0259 |
| Change (JSN) | 0.77 (0.95) | 0.15 (1.23) | 0.0208 |
| In patients with baseline JSW ≥3 mm | OAIL-1 (n=25) | OAnl (n=28) | |
| JSW (mm) at baseline | 4.67 (0.28) | 4.25 (0.19) | 0.142 |
| JSW (mm) at 24 months | 3.78 (0.35) | 3.86 (0.24) | 0.8203 |
| Change (JSN) | 0.89 (0.26) | 0.38 (0.18) | 0.0507 |
PBMC overexpression of TNFα also predicts OA progression
Analysis of the 86 “completer” patients also revealed that patients who are high expressors of IL-1β (OAIL-1) exhibit increased PBMC gene expression of TNFα (OATNFα) (significant positive correlation r=0.78, p <0.001). We therefore also stratified patients based on PBMC TNFα expression (QPCR) into OATNFα (overexpressors) and OAnl(TNF) (whose TNFα expression was comparable to controls). Similar to the OAIL-1 group, the OATNFα group demonstrated increased risk of progression in the signal knee compared to OAnl(TNF) group, as assessed by JSN greater than 30% (OR=8.9; 95% CI 2.57–30.81; p<0.0005).
Discussion
These studies demonstrate that PBMC gene expression in patients with symptomatic knee OA exhibits distinctive profiles. We identified a set of 173 differentially expressed genes, which have a sensitivity of 89% and specificity of 76% for the diagnosis of OA. In addition, we found that symptomatic knee OA patients can be segregated into two distinct classes based on levels of inflammatory gene expression (e.g., IL-1β, IL-8, COX-2). The differential over-expression of these inflammatory genes in OA subgroups was validated using QPCR (p<0.0001) in two cohorts from NYUHJD and one from Duke. In the cross-sectional analyses of the study populations, patients with the inflammatory gene “signature” (OAIL-1) had higher numbers of affected joints and higher pain scores. In a 24-month longitudinal study of the NYUHJD Validation Cohort, the OAIL-1 subclass also exhibited an increased risk for radiographic progression.
The present study is the first to comprehensively characterize the gene expression signature of peripheral blood cells isolated from patients with symptomatic and radiographically confirmed tibio-femoral knee OA. Previously, Marshall et al had identified a group of genes using a custom cDNA microarray constructed with genes expressed by chondrocytes; these were different genes than reported in the current study, and were classified by Marshall et al as “mild OA” detection markers (30). The discrepancy between that study and the present report may be due to the fact that Marshall studied early OA detected by arthroscopy, while our cohorts had more advanced disease and were likely to have more extensive synovial and bone lesions which would contribute to the state of PBMC gene activation.
As noted above, the BIPED system has been proposed for the classification of biomarkers in OA (13). Using the BIPED classification, we have identified candidates for three classes of genomic OA biomarkers: Diagnostic, Burden of disease and Prognostic. The Diagnostic transcriptome biomarker that statistically differentiated OA from control is detailed in supplemental data, and includes expression of genes such as JunB, MMP-9, TGFβ-1, lamin A/C, granulin, small inducible cytokine A3 and chemokine (c-c motif) receptor1. Protein products of these genes have been shown to play a role in cartilage degeneration and/or synovial tissue inflammation in OA (31-34). The potential Burden of disease biomarker was an inflammatory gene profile that identified two subclasses of OA patients, OAIL-1 and OAnl. Clinically, in a cross-sectional analysis, the OAIL-1 sub-group had higher numbers of joints affected and higher pain scores (both WOMAC and MDHAQ-VAS). Finally, this inflammatory gene expression profile also represents a candidate Prognostic biomarker. The OAIL-1 and related OATNF subclasses exhibited a 3- to 8-fold increased risk of radiographic progression at two years. As would be the case for all candidate biomarkers, these findings will require further replication, validation and qualification in larger longitudinal population cohorts.
An unanticipated finding of our study is that, while the OAIL-1 subset progressed more rapidly and had more pain at presentation, the mean JSW at baseline was higher than for OAnl. This raises the possibility that OAIL-1 may identify a patient subset with more aggressive OA, who exhibit an “inflammatory phenotype” in early disease (i.e., “early” as defined by greater JSW), but who are at higher risk for more rapid progression. We have planned future longitudinal studies to test this hypothesis.
Genomic biomarkers as described here are in distinction to most previously studied candidates in OA, typically biochemical markers of cartilage, bone or synovium degradation or synthesis, which have yielded mixed results as prognostic biomarkers (35-38). We (VBK) have recently demonstrated the strong correlation of total body burden of disease with several such biomarkers (sHA, sCOMP and uCTX2) (39); therefore, the correlation of such systemic biomarkers with local disease (e.g., knee) is limited by the total burden of OA in patients. The limitations of conventional synthesis/degradation biomarkers create a need to develop alternative strategies, such as those here described.
Our data are also of interest since they suggest a new approach to classify phenotypes of OA. To date the approach to the study of OA has not sufficiently taken diverse etiologies and phenotypes into account. Our studies provide a strategy that may allow the stratification of symptomatic knee OA patients based upon a PBMC transcriptome phenotype.
Recent studies have suggested an association between synovial inflammatory processes and the progression of structural changes in OA (40). OA synovial pannus is highly vascular and expresses cytokines such as IL-1β, iNOS and metalloproteinases (41), which suggests that it contributes to cartilage degradation. In addition, synovial proliferation in OA can be highly vascularized, providing a site where circulating leukocytes can be exposed to growth factors and cytokines. Recently, serum proteins such as macrophage inhibitory protein (MIP)-1α, IL-1α, IL-2, IL-15 and MMP-7 were shown to differentiate early OA from controls (42). Fraenkel et al have reported associations of IL-1β production with presence of knee osteophytes as well as joint space narrowing in women; these women also had higher rates of knee OA, but lower rates of hand OA (43). These data reinforce the notion that inflammation is important in subsets of patients with osteoarthritis. Furthermore, the literature is conflicting on the association of genetic variations among IL-1 family genes with OA severity and progression. However, recently Kerkhof et al (12) have reported from large scale meta-analysis that genetic variations in IL-1β did not correlate with OA incidence and severity. Additionally, we have also reported that genetic variations in interleukin-1-receptor antagonist (IL1RN) predicted radiographic KL scores in knee OA and were associated with lower odds of radiographic severity, greater joint space width, and lower synovial fluid cytokine levels (11).
In summary, our findings indicate that there is a subset of OA patients, “OAIL-1,” whose peripheral leukocytes are “activated.” The data suggest that PBMC gene expression profiles merit further validation as prognostic biomarkers in patients with knee OA.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health to Dr. Abramson (R01 AR052873) and Dr. Kraus (R01 AR048769 and U01 AR050898).
Footnotes
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Attur and Abramson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Attur, Kraus, Abramson.
Acquisition of data. Attur, Krasnokutsky, Greenberg, Samuels, Smiles, Lee, Patel, Al-Mussawir, McDaniel, Kraus, Abramson.
Analysis and interpretation of data. Attur, Belitskaya-Lévy, Oh, Krasnokutsky, Greenberg, Samuels, Kraus, Abramson.
Disclosure of Interest: Drs. Steven B. Abramson and Mukundan Attur have filed a patent on the use of IL-1-beta and TNF-alpha as markers for knee osteoarthritis progression.
All other authors have disclosed no conflict of interest.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Brief-Medical Technology. Hyattsville, MD, U.S.: Department of Health and Human Services, Centers for DiseaseControl and Prevention; 2010. Health, United States, 2009. http://www.cdc.gov/nchs/data/hus/hus09_InBrief_MedicalTech.pdf. [Google Scholar]
- 4.Kraus VB. Waiting for action on the osteoarthritis front. Curr Drug Targets. 2010;11:518–20. doi: 10.2174/138945010791011974. [DOI] [PubMed] [Google Scholar]
- 5.Attur MG, Dave M, Cipolletta C, Kang P, Goldring MB, Patel IR, et al. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J Biol Chem. 2000;275:40307–15. doi: 10.1074/jbc.M002721200. [DOI] [PubMed] [Google Scholar]
- 6.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Logar DB, Komadina R, Prezelj J, Ostanek B, Trost Z, Marc J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab. 2007;25:219–25. doi: 10.1007/s00774-007-0753-0. [DOI] [PubMed] [Google Scholar]
- 8.Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–44. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–9. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 10.Goekoop RJ, Kloppenburg M, Kroon HM, Frolich M, Huizinga TW, Westendorp RG, et al. Low innate production of interleukin-1beta and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage. 2010;18:942–7. doi: 10.1016/j.joca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Attur M, Wang HY, Kraus VB, Bukowski JF, Aziz N, Krasnokutsky S, et al. Radiographic severity of knee osteoarthritis is conditional on interleukin 1 receptor antagonist gene variations. Ann Rheum Dis. 2010;69:856–61. doi: 10.1136/ard.2009.113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkhof HJM, Doherty M, Arden NK, Abramson SB, Attur M, Bos SD, et al. Large-scale meta-analysis of interleukin-1 beta and interleukin-1 receptor antagonist polymorphisms on risk of radiographic hip and knee osteoarthritis and severity of knee osteoarthritis. Osteoarthritis Cartilage. 2010 Dec 10; doi: 10.1016/j.joca.2010.12.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14:723–7. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 16.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a MDHAQ [corrected] for standard care of patients with rheumatic diseases. J Rheumatol. 2005;32:1432–9. [PubMed] [Google Scholar]
- 17.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 18.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, et al. Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis. 2009;68:1673–9. doi: 10.1136/ard.2008.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyam A. Isolation of leukocytes from human blood. A two phase system for removal of red cells with ethylcellulose as erythrocyte-aggregating agent. Scand J Clin Lab Invest. 1968;21(Suppl 97):9–29. [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Bioconductor. Open source software for bioinformatics. www bioconductor org. 2009 Available from: URL: www.bioconductor.org.
- 23.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.The R language for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: R: A language and environment for statistical computing. Available from: URL: http://www.R-project.org. [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J Royal Stat Society Series B. 1995;57:289–300. [Google Scholar]
- 27.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99:6567–72. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibshirani RJ, Efron B. Pre-validation and inference in microarrays. Stat Appl Genet Mol Biol. 2002;1 doi: 10.2202/1544-6115.1000. Article1. [DOI] [PubMed] [Google Scholar]
- 30.Marshall KW, Zhang H, Yager TD, Nossova N, Dempsey A, Zheng R, et al. Blood-based biomarkers for detecting mild osteoarthritis in the human knee. Osteoarthritis Cartilage. 2005;13:861–71. doi: 10.1016/j.joca.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–9. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borzi RM, Mazzetti I, Macor S, Silvestri T, Bassi A, Cattini L, et al. Flow cytometric analysis of intracellular chemokines in chondrocytes in vivo: constitutive expression and enhancement in osteoarthritis and rheumatoid arthritis. FEBS Lett. 1999;455:238–42. doi: 10.1016/s0014-5793(99)00886-8. [DOI] [PubMed] [Google Scholar]
- 33.Benderdour M, Tardif G, Pelletier JP, Di Battista JA, Reboul P, Ranger P, et al. Interleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1beta. J Rheumatol. 2002;29:1262–72. [PubMed] [Google Scholar]
- 34.Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, et al. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–55. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 35.Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, et al. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004;50:2471–8. doi: 10.1002/art.20332. [DOI] [PubMed] [Google Scholar]
- 36.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio-Le Graverand MP, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007;15:379–85. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17:384–9. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Bingham CO, III, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 39.Kraus VB, Kepler TB, Stabler T, Renner J, Jordan J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS One. 2010;5(3):e9739. doi: 10.1371/journal.pone.0009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibakawa A, Aoki H, Masuko-Hongo K, Kato T, Tanaka M, Nishioka K, et al. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage. 2003;11:133–40. doi: 10.1053/joca.2002.0871. [DOI] [PubMed] [Google Scholar]
- 41.Krasnokutsky S, Samuels J, Attur M, Regatte R, Babb J, Rosenthal P, et al. Synovial but not cartilage volumes on MRI predict radiographic severity of knee OA. [abstract] Arthritis Rheum. 2008;58(Suppl):S889–S890. [Google Scholar]
- 42.Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17:43–8. doi: 10.1016/j.joca.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraenkel L, Roubenoff R, LaValley M, McAlindon T, Chaisson C, Evans S, et al. The association of peripheral monocyte derived interleukin 1beta (IL-1beta), IL-1 receptor antagonist, and tumor necrosis factor-alpha with osteoarthritis in the elderly. J Rheumatol. 1998;25:1820–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


